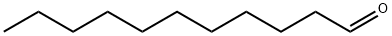Chemical Properties
Undecanal is a colorless liquid with a sweetish, fatty odor with an orange and rose undertone. It has a characteristic flavor. This chemical tends to polymerize unless tightly sealed. It is soluble in oils and alcohol, but insoluble in glycerol and water. Additionally, it is combustible.
Occurrence
Reported found in essential oils of citrus peels, lemon, caviar, cooked beef, chicken, lamb, pork, coriander leaf,
cucumber, fish, grapefruit juice, apple, orange juice, bilberry, cranberry, raspberry, blackberry, carrot, celery, baked potato, Gruyere
cheese, Russian cheese, butter, milk, fatty fish, hop oil, beer, cognac, tea, peanut oil, pecan, starfruit, coriander seed, rice, calamus,
buckwheat, red sage, loganberry and maté.
Uses
Undecanal is a flavoring agent that is a liquid, colorless or pale yellow,
with a sweet odor. it is soluble in most fixed oils, mineral oil, and
propylene glycol; insoluble in glycerin. it is obtained by chemical
synthesis. it is also termed aldehyde c-11 undecyclic and n-undecyl
aldehyde.
Preparation
Usually prepared by oxidation of the corresponding alcohol or reduction of the corresponding acid.
Definition
ChEBI: Undecanal is a saturated fatty aldehyde formally arising from reduction of the carboxylic acid group of undecanoic acid. It is a component of essential oils from citrus plants like Citrus reticulata. It has a role as an antimycobacterial drug, a volatile oil component and a plant metabolite. It is a saturated fatty aldehyde, a n-alkanal and a medium-chain fatty aldehyde. It is a tautomer of an undec-1-en-1-ol.
Aroma threshold values
Detection: 0.4 to 100 ppb
Taste threshold values
Taste characteristics at 10 ppm: waxy, buttery, aldehydic, soapy with a citrus note and slight laundry detergent
nuance.
General Description
Undecanal is a volatile flavor compound identified in grapefruit oil and orange essential oil. It is used as a pharmaceutical intermediate.
Hazard
Toxic by ingestion and inhalation, irritant
to tissue.
Flammability and Explosibility
Not classified
Safety Profile
Low toxicity by
ingestion and skin contact. A skin irritant.
Combustible liquid when exposed to heat or
flame. To fight fire, use CO2, dry chemical.
When heated to decomposition it emits
acrid smoke and irritating fumes. See also
ALDEHYDES.
References
C.M Paleos Angelos Malliaris. Chemical evidence concerning the solubilization site of undecanal in micelles. Journal of Colloid and Interface Science. 1981, 82 (1), 244-247. DOI:
10.1016/0021-9797(81)90145-4George W. Mushrush, Robert N. Hazlett, Harold G. Eaton. Liquid-phase oxidation of undecanal by tert-butyl hydroperoxide in dodecane. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24 (2), 290-293. DOI:
10.1021/I300018A023Kinetic Modeling of Rhodium‐Catalyzed Reductive Amination of Undecanal in Different Solvent Systems DOI:
10.1002/cite.201900135Chen Wen-kan. Synthesis of Undecanal by Selective Oxidation of Undecanol. Chemistry 2015