Synthesis
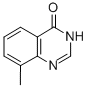
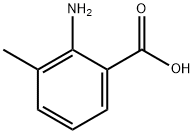
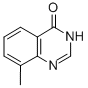
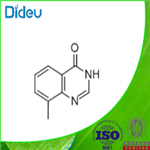
Formamide (26 mL, 0.6600 mol) was added to 2-amino-3-methylbenzoic acid (100 g, 0.66 mol) and formamidine acetate (206 g, 1.98 mol) in a 2L round-bottomed flask equipped with a mechanical stirrer. The reaction mixture was heated at 160 °C for 16 h. The reaction process was monitored by LCMS. After completion of the reaction, the mixture was cooled to room temperature and diluted with 2N NaOH solution (300 mL). After stirring for 15 min at room temperature, the reaction mixture was neutralized with 1.5N HCl solution. The precipitated solid product was collected by filtration, washed with ice water and dried under vacuum to afford 8-methyl-4-quinazolone (90 g, 86% yield) as an off-white solid. The structure of the product was confirmed by 1H NMR (DMSO-d6, 400 MHz): δ 12.21 (bs, 1H), 8.10 (s, 1H), 7.95-7.93 (dd, J = 8.8, 7.9 Hz, 1H), 7.65-7.63 (d, J = 7.9 Hz, 1H), 7.39-7.35 (t, J = 15.2 Hz, 1H ), 2.51 (s, 3H).
References
[1] Patent: WO2013/96194, 2013, A1. Location in patent: Page/Page column 28-29
[2] European Journal of Medicinal Chemistry, 2012, vol. 50, p. 264 - 273
[3] Journal of Medicinal Chemistry, 2016, vol. 59, # 6, p. 2794 - 2809
[4] Chemische Berichte, 1905, vol. 38, p. 3555
[5] Journal of Organic Chemistry, 1952, vol. 17, p. 149,153