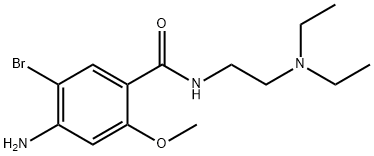
Description
Bromopride is a dopamine D
2 receptor antagonist (K
i = 14 nM). It binds to bovine anterior pituitary membranes with an IC
50 value of 2.13 μM. Bromopride inhibits cholinesterase activity
in vitro in rat plasma and striatum homogenate (IC
50s = 67.8 and 38.4 μM, respectively). It increases serum prolactin levels in rats by 100% when used at a dose of 0.02 mol/kg. Bromopride (2.5 mg/kg) decreases locomotion and rearing in an open field test and impairs acquistion, but not retention, of a conditioned avoidance response in a two-way active conditioned avoidance test in rats.
Chemical Properties
White Solid
Originator
Praiden,Italchemi,Italy,1977
Uses
An antiemetic.Bromopride is used in method and a system for analyzing neuropharmacology of a drug.
Definition
ChEBI: 4-amino-5-bromo-N-[2-(diethylamino)ethyl]-2-methoxybenzamide is a member of benzamides.
Manufacturing Process
To 119 g (0.45 mol) of N-(2-diethylaminoethyl)-2-methoxy-4-aminobenzamide
dissolved in 200 cc of acetic acid are added in the cold in small portions 69 g
of acetic anhydride (0.45 mol + 50% excess). The starting material is made
by esterifying 4-aminosalicylic acid with methanol, then acetylating with acetic
anhydride and then methylating with dimethyl sulfate. The solution obtained is
heated for 2 hours on a water bath and then boiled for 15 minutes. It is
cooled at 25°C. While agitating constantly and maintaining the temperature
between 25° and 30°C, there is added to the solution drop by drop 72 g of
bromine dissolved in 60 cc of acetic acid. It is agitated for one hour. The mixture obtained is added to one liter of water and the base is precipitated by
the addition of 30% soda, The precipitated base is extracted with 40 cc of
methylene chloride. After evaporation of the solvent, the residue is boiled for
two hours with 390 g of concentrated hydrochloric acid in 780 cc of water. It
is cooled, diluted with one liter of water, 12 g of charcoal are added, and the
mixture filtered. The base is precipitated with 30% soda. The N-(2-
diethylaminoethyl)-2-methoxy-4-amino-5-bromobenzamide formed
crystallizes, is centrifuged and washed with water. A yield of 85 g of base
having a melting point of 129°-130°C is obtained.
To produce the dihydrochloride, the free base is dissolved in 110 cc of
absolute alcohol, 9.6 g of dry hydrochloric acid dissolved in 35 cc of alcohol
are added, followed by 2.8 cc of water. The dihydrochloride precipitates, is
centrifuged, washed, and dried at 40°C. It was a solid white material having a
melting point of 134°-135°C.
Therapeutic Function
Antiemetic
References
[1] M. TONINI. Clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics[J]. Alimentary Pharmacology & Therapeutics, 2004, 19 4: 379-390. DOI:
10.1111/j.1365-2036.2004.01867.x[2] HERBERT Y. MELTZER Rebecca S Miljana Simonovic. Effects of a series of substituted benzamides on rat prolactin secretion and 3H-spiperone binding to bovine anterior pituitary membranes[J]. Life sciences, 1983, 32 25: Pages 2877-2886. DOI:
10.1016/0024-3205(83)90324-7[3] A G NASELLO. Differential effects of bromopride and domperidone on cholinesterase activity in rat tissues.[J]. Life sciences, 1995, 56 3: 151-156. DOI:
10.1016/0024-3205(94)00429-v[4] ANTONIA GLADYS NASELLO Luciano F F Maria Luzinete Alves Vanzeler. A Comparison of Bromopride and Domperidone Effects on Rat Conditioned Avoidance and Motor Activity[J]. Basic & Clinical Pharmacology & Toxicology, 1991, 68 1: 46-50. DOI:
10.1111/j.1600-0773.1991.tb01206.x