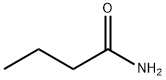
Uses
Butyramide was used in the synthesis of hydroxamic acids, electrorheological fluids and β-amodoorganotin compounds. It was used as substrate of (+)-γ-lactamase to develop a microreactor to study enzyme stability, activity, kinetics and substrate specificity.
Preparation
Butyramide is prepared by amination of n-butyric acid. n-Butyric acid is heated with ammonia gas bubbled through it, and the reaction mixture reaches 200–215°C after approximately 40 hours. Heating and ammonia flow are then stopped, and the mixture is cooled below 20°C before filtration to yield butyramide.
An alternative method involves reacting n-butyric acid with urea: First, heat a mixture of 8 kg of n-butyric acid and 3 kg of urea under reflux for 10 hours. Allow the mixture to cool to 70–80°C, then add a suitable amount of hot ethanol. Decolorize the hot solution, filter it while hot, and cool. The crude product obtained by filtration is recrystallised from ethanol to yield the final product.