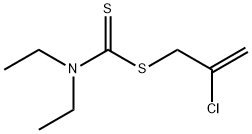
Chemical Properties
Sulfallate is an amber liquid.
Uses
Sulfallate is a selective pre-planting or pre-emergence herbicide.
Definition
ChEBI: Sulfallate is a thiocarbonyl compound.
General Description
Amber to dark amber liquid.
Air & Water Reactions
Insoluble in water. Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.
Reactivity Profile
SULFALLATE is incompatible with strong oxidizing agents .Also incompatible with alkalis. .
Fire Hazard
SULFALLATE is probably combustible.
Safety Profile
Confirmed carcinogen
with experimental carcinogenic data.
Moderately toxic by ingestion and skin
contact. Mutation data reported. An
herbicide. When heated to decomposition it
emits very toxic fumes of Cl-, NOx, and
SOx. See also ALLYL COMPOUNDS,
CARBAMATES, and ESTERS.
Potential Exposure
A dithiocarbamate. The major use for sulfallate in the United States is as a preemergent selective herbicide to control certain annual grasses and broadleaf weeds around vegetable and fruit crops. Sulfallate has also been used for weed control among shrubbery and ornamental plants. Some dithiocarbamates have been used as rubber components
Carcinogenicity
Sulfallate is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN2771 Dithiocarbamate and Thiocarbamate pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Dithiocarbamate esters are combustible. They react violently with powerful oxidizers such as calcium hypochlorite. Poisonous gases are generated by the thermal decomposition of dithiocarbamate compounds, including carbon disulfide, oxides of sulfur, oxides of nitrogen, hydrogen sulfide, ammonia, and methylamine.Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of dithiocarbamate with aldehydes, nitrides, and hydrides. Dithiocarbamate are incompatible with acids, peroxides, and acid halides.
Waste Disposal
Small amounts may be decomposed by strong oxidizing agent. Large amounts should be incinerated in a unit with effluent gas scrubbing.