Description
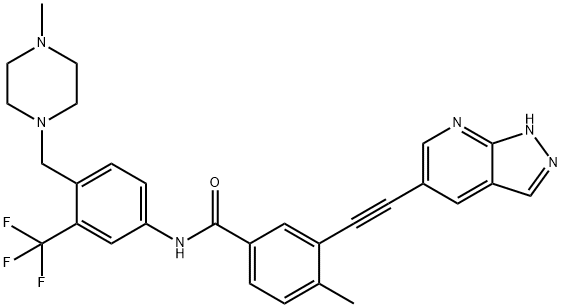
GZD-824 is an orally available inhibitor of a broad spectrum of Bcr/Abl tyrosine kinase mutants including T315I (IC
50s = 0.34 and 0.68 nM for wild-type Bcr/Abl and Bcr/Abl
T315I, respectively). It has been shown to suppress the proliferation of Bcr/Abl-positive K562 and Ku812 human chronic myelogenous leukemia cells (IC
50s = 0.2 and 0.13 nM, respectively) and induce tumor regression in mouse xenograft tumor models driven by either wild-type or mutant Bcr/Abl.
Uses
GZD824 is a orally bioavailable inhibitor that targets phosphorylated and non-phosphorylated Breakpoint Cluster Region-Abelson (Bcr-Abl) kinases. It is a COVID19-related research product.
Definition
GZD 824 (Olverembatinib), another third-generation BCR-ABL tyrosine kinase inhibitor (TKI), was approved in 2021 in China for patients with T315I positive chronic phase chronic myeloid leukemia (CML-CP) or accelerated-phase CML (CML-AP). Like ponatinib, olverembatinib inhibits multikinases, including B-RAF, DDR1, FGFR, Flt3, KIT, PDGFRα/β, RET, and SRC.
General Description
Class: non-receptor tyrosine kinase
Treatment: CML
Elimination half-life = 17.5–36.5 h