Description
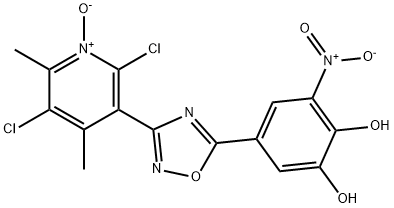
Opicapone is a selective and
reversible catechol O-methyltransferase (COMT) inhibitor that
was developed by the Portuguese pharmaceutical firm Bial and
sold to Neurocrine Biosciences. The drug was approved by
the USFDA as adjunctive treatment to levodopa (L-Dopa)/
dopa-decarboxylase inhibitor (DDCI) therapy in adults with
Parkinson’s disease (PD) and end-of-dose motor fluctuations
that cannot be stabilized on those combinations. In 14- to 15-
week double-blind multinational trials and in one-year openlabel
extension studies in this patient population, opicapone
was an effective and generally well-tolerated adjunctive therapy
to L-Dopa plus a DDCI and other PD therapies. During the
double-blind phase, adjunctive opicapone (50 mg once daily)
provided significantly greater improvements in motor fluctuations
than placebo, and no new unexpected safety concerns
were identified after treatment with opicapone over a 1.4 year
period. Furthermore, no serious cases of hepatotoxicity were
reported in clinical trials, which represents a significant safety
profile improvement over existing standard-of-care COMT
inhibitors enticapone, tolcapone, and nebicapone.
Uses
Opicapone, is used for the synthesis of novel nitrocatechol-substituted heterocycles, having the ability to inhibit catechol-O-methyltransferase (COMT), used for the treatment of Parkinson`s diseases.
Definition
ChEBI: Opicapone is a ring assembly and an oxadiazole.
Synthesis
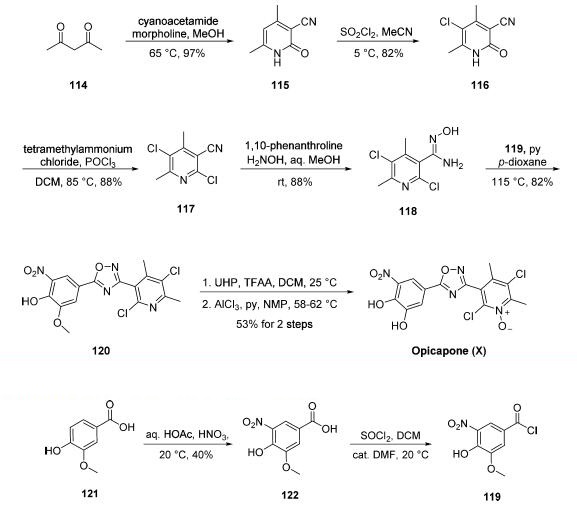
Although several synthetic approaches to opicapone or
opicapone subunits have been disclosed, a synthetic approach
described by Bial was exemplified on a scale capable of
producing 14.4 kg of the active pharmaceutical ingredient
(API). Commercial 2,4-pentanedione (114) was condensed
with cyanoacetamide in warm methanol to give rise to
cyanopyridone 115 in excellent yield. Chlorination
with sulfuryl chloride in chilled acetonitrile followed by
treatment with phosphorus oxychloride resulted in dichloropyridine
117. Next, treatment with hydroxylamine in aqueous
methanol converted nitrile 117 to the corresponding Nhydroxyamidine
118, and this was followed by exposure to
pyridine and acid chloride 119. These operations facilitated a cyclization
reaction, which furnished the key oxadiazole 120 in good yield.
Subjection of 120 to urea hydrogen peroxide (UHP) in
dichloromethane to establish the pyridine N-oxide functionality
within opicapone preceded methyl ether cleavage through the
use of aluminum trichloride in warm pyridine to furnish
opicapone (X) in 53% yield for the two-step sequence.
The preparation of acid chloride 119 involved the nitration
of commercially available benzoic acid 121 followed by thionyl
chloride-mediated conversion of the resulting nitrobenzoic acid
122 to acid chloride 119. Interestingly, although
the nitration step is low-yielding and involves nitric acid, the
authors report an operationally simple isolation method that
has been exemplified on multiple kilogram scale. No yield was
reported for the conversion of 122 to 119.