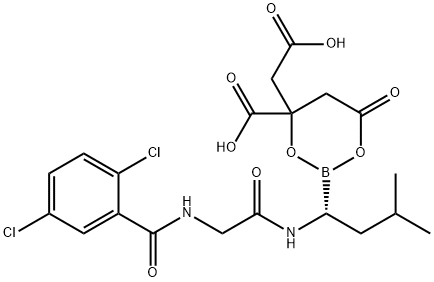
Description
Ixazomib citrate is a
proteasome inhibitor prodrug for the treatment of multiple
myeloma in patients who have received at least one prior
therapy in combination with lenalidomide and dexamethasone. The drug was developed by Takeda and reversibly
inhibits the protein proteasome subunit β type-5, which is part
of the 20S proteasome complex. Ixazomib citrate (XXIV) is
hydrolyzed quickly in vivo to give the biologically active
compound ixazomib, which presumably is the corresponding
boronic acid variant of XXIV.
Uses
MLN-9708 is a novel proteasomeinhibitor.
Clinical Use
Highly selective and reversible proteasome inhibitor:
Treatment of multiple myeloma in combination with
lenalidomide and dexamethasone
Synthesis
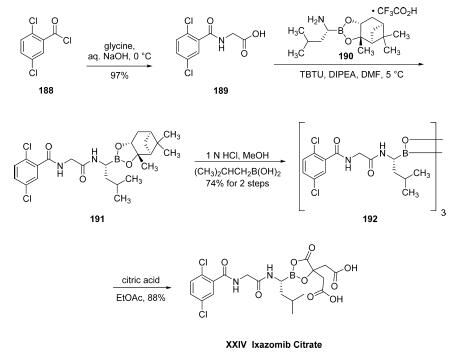
The structure of ixazomib citrate is particularly interesting in
that it is one of the relatively few marketed drugs which feature
a boron atom within its structure (others of note being the
oncology medication bortezomib and the antifungal drug
tavaborole13). The ostensible scale synthetic approach began
with reaction of commercial 2,5-dichlorobenzoyl chloride (188) with glycine in aqueous NaOH to furnish amide
189 in 97% yield as a white crystalline solid. Acid 189 was then
coupled with commercially available 1,3,2-benzodioxaborolane
190 in the presence of TBTU and DIPEA in DMF at low
temperature to give diamide 191, which was used without
purification for the next step. Borane 191 was then deprotected
with (2-methylpropyl)boronic acid in methanolic HCl to
provide trimer 192 in 74% as a white solid. Finally, boroxin
192 was reacted with citric acid in EtOAc to dissociate the
trimer, resulting in ixazomib citrate (XXIV) in 88% yield as a
crystalline solid.
References
[1] Patent: WO2009/154737, 2009, A1. Location in patent: Page/Page column 63