Chemical Properties
Propylene glycol is a clear, colorless, viscous, practically odorless
liquid, with a sweet, slightly acrid taste resembling that of glycerin.
Chemical Properties
Colorless to light yellow liqui
Uses
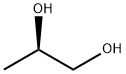
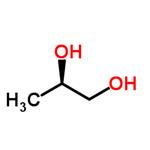
R-(-)-1,2-Propanediol, is a chiral building blocks that can be used for variety of chemical synthesis.
Production Methods
Propylene is converted to chlorohydrin by chlorine water and
hydrolyzed to 1,2-propylene oxide. With further hydrolysis, 1,2-
propylene oxide is converted to propylene glycol.
Definition
ChEBI: (R)-propane-1,2-diol is a propane-1,2-diol. It has a role as a human metabolite and an Escherichia coli metabolite. It is an enantiomer of a (S)-propane-1,2-diol.
Pharmaceutical Applications
Propylene glycol has become widely used as a solvent, extractant,
and preservative in a variety of parenteral and nonparenteral
pharmaceutical formulations. It is a better general solvent than
glycerin and dissolves a wide variety of materials, such as
corticosteroids, phenols, sulfa drugs, barbiturates, vitamins (A
and D), most alkaloids, and many local anesthetics.
As an antiseptic it is similar to ethanol, and against molds it is
similar to glycerin and only slightly less effective than ethanol.
Propylene glycol is commonly used as a plasticizer in aqueous
film-coating formulations.
Propylene glycol is also used in cosmetics and in the food
industry as a carrier for emulsifiers and as a vehicle for flavors in
preference to ethanol, since its lack of volatility provides a more
uniform flavor.
Safety
Propylene glycol is used in a wide variety of pharmaceutical
formulations and is generally regarded as a relatively nontoxic
material. It is also used extensively in foods and cosmetics. Probably
as a consequence of its metabolism and excretion, propylene glycol
is less toxic than other glycols. Propylene glycol is rapidly absorbed
from the gastrointestinal tract; there is also evidence that it is
absorbed topically when applied to damaged skin. It is extensively
metabolized in the liver, mainly to lactic and pyruvic acids, and is
also excreted unchanged in the urine.
In topical preparations, propylene glycol is regarded as
minimally irritant,although it is more irritant than glycerin.
There have been some reports of contact dermatitis associated with
propylene glycol.Some local irritation is produced upon
application to mucous membranes or when it is used under
occlusive conditions.Parenteral administration may cause pain
or irritation when propylene glycol is used in high concentration.
Propylene glycol is estimated to be one-third as intoxicating as
ethanol, with administration of large volumes being associated with
adverse effects most commonly on the central nervous system,
especially in neonates and children.Other adverse reactions
reported, though generally isolated, include: ototoxicity;cardiovascular
effects; seizures; and hyperosmolarity and lactic
acidosis, both of which occur most frequently in patients with
renal impairment. Adverse effects are more likely to occur following
consumption of large quantities of propylene glycol or on
adminstration to neonates, children under 4 years of age, pregnant
women, and patients with hepatic or renal failure. Adverse events
may also occur in patients treated with disulfiram or metronidazole.
On the basis of metabolic and toxicological data, the WHO has
set an acceptable daily intake of propylene glycol at up to 25 mg/kg
body-weight.Formulations containing 35% propylene glycol
can cause hemolysis in humans.
In animal studies, there has been no evidence that propylene
glycol is teratogenic or mutagenic. Rats can tolerate a repeated oral
daily dose of up to 30 mL/kg body-weight in the diet over 6 months,
while the dog is unaffected by a repeated oral daily dose of 2 g/kg in
the diet for 2 years.
(mouse, IP): 9.72 g/kg
(mouse, IV): 6.63 g/kg
(mouse, oral): 22.0 g/kg
(mouse, SC): 17.34 g/kg
(rat, IM): 0.01 g/kg
(rat, IP): 6.66 g/kg
(rat, IV): 6.42 g/kg
(rat, oral): 0.02 g/kg
(rat, SC): 22.5 g/kg
storage
At cool temperatures, propylene glycol is stable in a well-closed
container, but at high temperatures, in the open, it tends to oxidize,
giving rise to products such as propionaldehyde, lactic acid, pyruvic
acid, and acetic acid. Propylene glycol is chemically stable when
mixed with ethanol (95%), glycerin, or water; aqueous solutions
may be sterilized by autoclaving.
Propylene glycol is hygroscopic and should be stored in a wellclosed
container, protected from light, in a cool, dry place.
Incompatibilities
Propylene glycol is incompatible with oxidizing reagents such as
potassium permanganate.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (dental
preparations; IM and IV injections; inhalations; ophthalmic, oral,
otic, percutaneous, rectal, topical, and vaginal preparations).
Included in nonparenteral and parenteral medicines licensed in the
UK. Included in the Canadian List of Acceptable Non-medicinal
Ingredients.