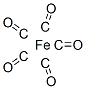Chemical Properties
Iron pentacarbonyl reacts with cyclopentadiene at 135° in an autoclave to give the
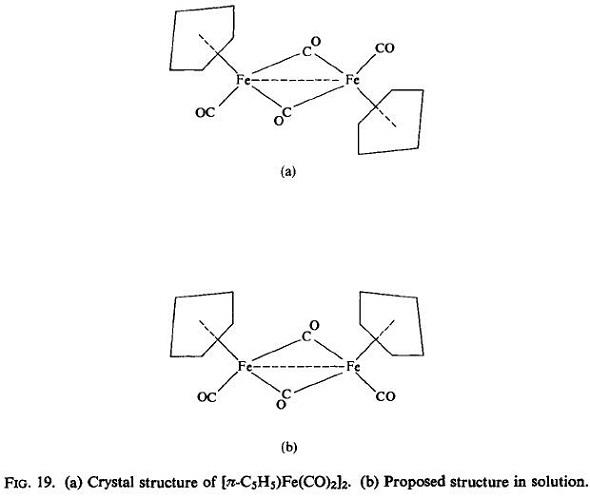
binuclear complex [(π-C5H5)Fe(CO)2]2 as deep reddish-purple crystals which are stable to
air and water. The crystal structure determination shows this molecule to be centrosymmetric with two bridging carbonyls forming a plane with the two iron atoms [Fig. 19(a)]. The rather short Fe-Fe distance of 2-49 ? and the diamagnetism of the compound indicate
the presence of the Fe-Fe bond. In solution, however, it tautomerizes to the structure believed to be that shown in Fig.19(b),in which the two rings lie on one side of the mean
plane formed by the bridging carbonyl groups and the iron atoms and the two terminal CO
groups lie on the other side.
Chemical Properties
Mobile, yellow liquid.Evolves carbon monoxide on exposure to air or light. Soluble in nickel
tetracarbonyl and most organic solvents; soluble
with decomposition in acids and alkalies; insoluble in water.
Uses
Catalyst in organic reactions, carbonyl iron for
high-frequency coils.
Uses
Iron pentacarbonyl is a useful dehalogenating and carbonylating agent.
Production Methods
Although nickel carbonyl can be obtained from nickel and carbon monoxide at atmospheric pressure and moderate temperature, the production of iron pentacarbonyl requires a pressure of 5– 30 MPa, a temperature of 150–200 C, and the presence of reactive iron. Even at high temperature and pressure, massive iron reacts sluggishly with carbon monoxide, so iron sponge, with its greater surface area, is used as starting material.
Hazard
Flammable, dangerous fire risk. Toxic by
ingestion, inhalation, and skin absorption.
Chemical Reactivity
Iron pentacarbonyl is an easily combustible substance. It does not react with water or with weak or dilute acids. With concentrated acids, the corresponding iron salts are formed with the evolution of carbon monoxide and hydrogen. Reactions with halogens yield iron halides. Iron pentacarbonyl also reduces organic compounds; for example, nitrobenzene is reduced to aniline; ketones to alcohols; and indigo to indigo white.
Structure and conformation
The trigonal
bipyramidal structure has been preferred to the square pyramidal one in the interpretation of electron diffraction, Raman and infrared and thermodynamic data; the apparently
contradictory dipole moment of 0-8 D being attributed to atom polarization. A single
crystal X-ray structure determination at - 80°C has confirmed the trigonal bipyramidalstructure.