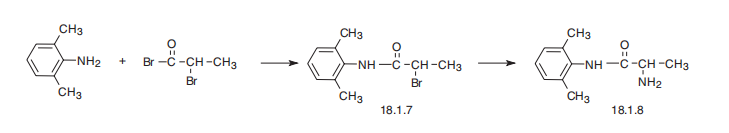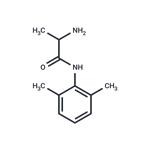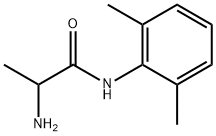
TOCAINIDE
- Product NameTOCAINIDE
- CAS41708-72-9
- CBNumberCB8210193
- MFC11H16N2O
- MW192.26
- EINECS255-505-0
- MDL NumberMFCD00072010
- MOL File41708-72-9.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 246-266 °C |
| Boiling point | 328.25°C (rough estimate) |
| Density | 1.0529 (rough estimate) |
| refractive index | 1.5750 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| form | Solid |
| pka | pKa 7.75(H2O t = 25.0±0.2 I = 0.01 (NaCl)) (Uncertain) |
| color | White to off-white |
| CAS DataBase Reference | 41708-72-9(CAS DataBase Reference) |
| FDA UNII | 27DXO59SAN |
| ATC code | C01BB03 |
Safety
| Symbol(GHS) |

|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| Hazard Codes | Xn |
| Risk Statements | 22 |