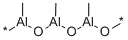Description
Methylaluminoxane (MAO) is by far the industrially most significant aluminoxane. The direct hydrolysis of trimethylaluminum (TMA) with water, however, is extremely exothermic and difficult to control. Thus, small scale, continuous processes, rather than large scale batch processes, are used for the direct hydrolysis of TMA to MAO. MAO can also be produced by reaction of TMA with organic acids, ketones, or carbon dioxide. The synthesis of MAO from TMA and CO2 actually has substantial literature precedent. Carbonation of an organoaluminum compound with carbon dioxide forms an aluminum carboxylate.
Mechanism of action
The role of Methylaluminoxane in metallocene catalyst polymerization is fourfold. First, it alkylates the transition metal. Second, it extracts an alkyl group from the transition metal to generate an active cationic polymerization catalyst. Third, it acts as a bulky, inert anion to the highly reactive transition metal cation. Fourth, it scavenges impurities from the polymerization reactor.