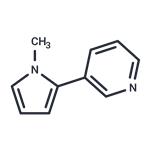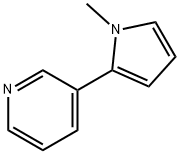
B-NICOTYRINE
- Product NameB-NICOTYRINE
- CAS487-19-4
- CBNumberCB8154891
- MFC10H10N2
- MW158.2
- EINECS207-651-1
- MDL NumberMFCD00468104
- MOL File487-19-4.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 168-169 °C |
| Boiling point | 78-80°C/0/15mm |
| Density | 1.2410 |
| refractive index | 1.6057 (estimate) |
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| solubility | Chloroform (Slightly), Dichloromethane (Slightly), Ethyl Acetate (Slightly), Met |
| form | Oil |
| pka | pK1:4.76(+1) (25°C) |
| color | Pale Yellow to Brown to Dark Orange |
| Stability | Light, Air Sensitive |
| FDA UNII | XN4R1LH79Y |
| EPA Substance Registry System | Pyridine, 3-(1-methyl-1H-pyrrol-2-yl)- (487-19-4) |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H319-H335-H302+H312+H332-H315 | |||||||||
| Precautionary statements | P280-P301+P312-P362+P364 | |||||||||
| HS Code | 2939800000 | |||||||||
| NFPA 704: |
|