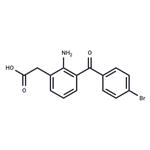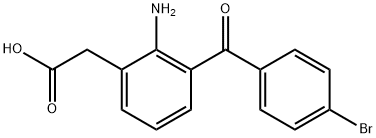
Bromfenac sodium
- Product NameBromfenac sodium
- CAS91714-94-2
- CBNumberCB8117032
- MFC15H12BrNO3
- MW334.16
- MDL NumberMFCD00864341
- MOL File91714-94-2.mol
Chemical Properties
| Boiling point | 562.2±50.0 °C(Predicted) |
| Density | 1.565±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| pka | 4.07±0.10(Predicted) |
| EWG's Food Scores | 1 |
| FDA UNII | 864P0921DW |
| ATC code | S01BC11 |
Bromfenac sodium Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| TRC A594895 | 50mg | $60 | 2-(2-Amino-3-(4-bromobenzoyl)phenyl)aceticAcid |
Buy |
| TRC A594895 | 200mg | $155 | 2-(2-Amino-3-(4-bromobenzoyl)phenyl)aceticAcid |
Buy |
| Biosynth Carbosynth FB66513 | 100mg | $150 | Bromfenac |
Buy |
| Biosynth Carbosynth FB66513 | 25mg | $60 | Bromfenac |
Buy |
| American Custom Chemicals Corporation API0009095 | 250MG | $222.6 | BROMFENAC 95.00% |
Buy |
Bromfenac sodium Chemical Properties,Usage,Production
Originator
Duract,Wyeth-AyerstUses
Bromfenac (Xibrom, ISTA Pharmaceuticals, Irvine, USA; Bronuck, Senju Pharmaceutical, Osaka, Japan) is indicated for the treatment of postoperative inflammation and the reduction of ocular pain in patients after undergoing cataract extraction. For this task, one drop of Xibrom may be applied to the affected eye twice daily beginning 24 hours after cataract surgery and continuing for the first 2 weeks of the postoperative period. The clinical safety and efficacy of bromfenac have been extensively studied in diverse comparative investigations, including the treatment of external or anterior ocular inflammatory diseases, allergic conjunctivitis, scleritis, and postoperative inflammation.The results of two phase III multicenter, randomized double-masked placebo-controlled clinical trials showed that bromfenac ophthalmic solution 0.09% was effective in the rapid resolution of ocular pain after cataract surgery, and there was a statistically significant difference between the bromfenac and placebo groups demonstrated in these phase III clinical trials.Uses
Analgesic.Definition
ChEBI: Amfenac in which the the hydrogen at the 4 position of the benzoyl group is substituted by bromine. It is used for the management of ocular pain and treatment of postoperative inflammation in patients who have undergone cataract extraction. It was withdraw from the US market in 1998, following concerns over off-label abuse and hepatic failure.Manufacturing Process
Reaction of (2-aminophenyl)-(4-bromophenyl)-methanone with methylsulfanylacetic acid ethyl ester and tert-butyl hypochlorite gives a corresponding sulfonium salt. This salt was transformed to initially to the betaine. Electrocyclic rearrangement of that transient intermediate leads, after rearomatization, to the homoanthranilic acid. Internal ester-amine interchange leads then to 4-bromophenyl-(3-(methylthio)indolin-7-yl)methanone. The thiomethyl group is then removed with Raney nickel to give 4-bromophenyl- (indolin-7-yl)methanone. Saponification of this intermediate affords the (2- amino-3-(4-bromobenzoyl)-phenyl)-acetic acid (Bromfenac).In practice it is usually used as sodium salt.
brand name
Xibrom (Ista).Therapeutic Function
Analgesicá AntiinflammatoryPharmacology
The drug is rapidly absorbed and excreted. The drug’s long duration of anti-inflammatory action despite its short half-life deserves further investigation.Clinical Use
Bromfenac, used as the sodium salt, is a non-steroidal anti-inflammatory drug acting via inhibition of COX-1 and COX-2. It was launched in Japan in 2000 by Senju for topical treatment of ocular inflammation and has been approved in the United States in 2006 for the treatment of pain following cataract surgery. It was used for the short-term treatment of acute pain, but it was withdrawn for this indication in June 1998 because of several postmarketing reports of severe hepatic failureSynthesis
The cyclization of 2-amino-4’- bromobenzophenone with ethyl 2-(methylthio) acetate with tert-butyl hypochlorite as catalyst in dichloromethane at 70 ?C gives 7- (4-bromobenzoyl)-3-(methylthio)-2,3-dihydro- 1H-indol-2-one, which is desulfurized by treatment with Raney nickel in THF yielding 7-(4- bromobenzoyl)-2,3-dihydro-1H-indol-2-one. Finally, this compound is hydrolyzed with refluxing 3 M aqueous NaOH and acidified with concentrated HCl .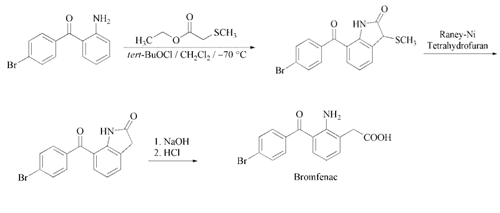
Veterinary Drugs and Treatments
Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) by virtue of its ability to block prostaglandin synthesis by inhibiting cyclooxygenase 1 and 2. Bromfenac is indicated for treatment of postoperative inflammation in patients who have undergone cataract extraction.Preparation Products And Raw materials
Bromfenac sodium Suppliers
Global(82)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29866 | 58 | |
| +86-755-89396905 +86-15013857715 |
admin@nexconn.com | China | 10406 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 | |
| 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 | |
| +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 | |
| +8615604608665 15604608665 |
dominicguo@gk-bio.com | CHINA | 9414 | 58 | |
| +86-852-30606658 | market18@leapchem.com | China | 24727 | 58 | |
| +86-85511178; +86-85511178; |
peter68@ptchemgroup.com | China | 35425 | 58 | |
| +86-18621343501; +undefined18621343501 |
product@acmec-e.com | China | 33338 | 58 | |
| +86-2552131256 +86-18251840740 |
sales@bicbiotech.com | China | 5994 | 58 |
View Lastest Price from Bromfenac sodium manufacturers
91714-94-2, Bromfenac sodiumRelated Search
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine