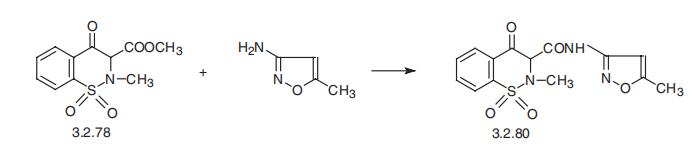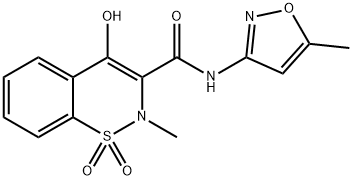
ISOXICAM
- Product NameISOXICAM
- CAS34552-84-6
- CBNumberCB8101486
- MFC14H13N3O5S
- MW335.34
- EINECS252-084-5
- MDL NumberMFCD00079374
- MOL File34552-84-6.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 265-271℃ |
| Density | 1.588 |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly, Heated), DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) |
| form | Solid |
| pka | 4.50±1.00(Predicted) |
| color | White to Pale Beige |
| FDA UNII | 8XU734C4NG |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |