Originator
Sieromicin,Sierochimica,Italy,1962
Uses
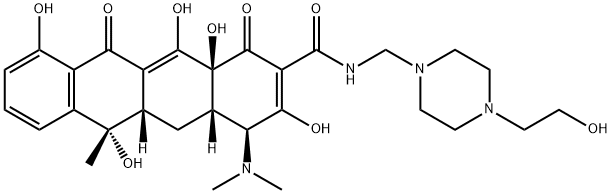
Pipacycline (mepicycline) is a semi-synthetic tetracycline formed by a Mannich condensation of formaldehyde and 4-hydroxyethylpiperazine with tetracycline. The introduction of the piperazine improves bioavailability, but Mannich bases are pro-drugs, converting back to the parent compound. Pipacycline is used commercially as a salt in combination with penicillin V for parenteral use (penimepicycline). The intrinsic in vitro activity and SARs for the amide region of the tetracycline molecule have not investigated extensively. Pipacycline has not been extensively cited in the literature.
Manufacturing Process
1.55 g p-formaldehyde were added to a solution of 7 g N-(β-hydroxyethyl)- diethylene diamine in 150 cc isopropanol and the whole was heated to 60°C for 30 minutes, to obtain complete dissolution; after cooling the solution to 40°C, 22.2 g of anhydrous tetracycline base were added as a fine powder and the reaction was allowed to proceed for 3 hours with agitation and while passing through a current of dry nitrogen; the solution was then filtered on a Buchner funnel and the filter cake was washed twice with 20 cc isopropanol; the crystalline cake was resuspended in 100 cc anhydrous ether, again filtered and washed 3 times with 50 cc anhydrous ether; finally, it was dried in vacuo and 28.6 g of product were obtained, namely a yield of 98%.
The characteristics of this product are as follows. It is a pale yellow, nonodorous, slightly bitter, crystalline powder, very soluble in water (>1.5 g/cc), soluble in methanol and formamide, slightly soluble in ethanol and isopropanol, insoluble in ether, benzene and chloroform; MP 162° to 163°C with decomposition (uncorrected).