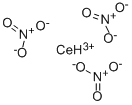
Physical properties
Hexahydrate is a colorless crystal; hygroscopic; loses water on heating— three molecules of water of crystallization expelled at 150°C; decomposes at 200°C; readily dissolves in water, alcohol, and acetone.
Uses
Cerium trinitrate is used for separation of Ce from other rare earths; catalyst in hydrolysis of phosphoric acid esters; mixture with Th(NO3)4 (1:99) formerly used in manufacture of incandescent mantles.
Preparation
Cerium(III) nitrate may be prepared by the action of nitric acid on a cerium(III) salt, followed by crystallization:
Ce
2(CO
3)
3 + 6HNO
3 → 2Ce(NO
3)
3 + 3CO
2 + 3H
2O.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intravenous
and intraperitoneal routes. Moderately toxic
by ingestion. Experimental reproductive
effects. See also CERIUM COMPOUNDS
and NITRATES. When heated to
decomposition it emits toxic fumes of NOx.