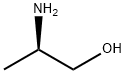
Chemical Properties
Colorless to light yellow liqui
Uses
(R)-(-)-2-Amino-1-propanol has been used as a derivatizing agent for gossypol during the analysis of gossypol enantiomers in cottonseed by high-performance liquid chromatography.
Application
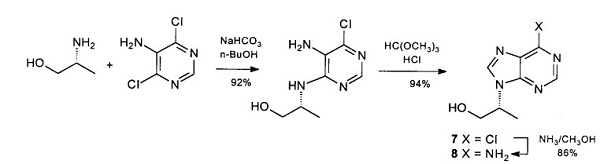
R)-(-)-2-Amino-1-propanol is used as a chiral intermediate for the synthesis of pharmaceutical products and agrochemicals. It acts as additives in health care products and animal feed. It is a amino alcohol
obtained by reduction of d-alanine. This compound could be converted into the corresponding nonracemic
adenine derivative (R)-9-(1-Methyl-2-hydroxyethyl)adenine through minor variations on the classic
Montgomery sequence[1].
General Description
(
R)-(-)-2-Amino-1-propanol is a chiral secondary amine.
Synthesis
General procedure for the synthesis of (R)-2-aminopropan-1-ol from D-alanine: The present embodiment relates to the synthesis of a class of intermediates of long-chain ethylpiperazine sulfonamide derivatives, in particular the preparation of (R)-2-aminopropan-1-ol. 150 mL of anhydrous THF and 1.1 g of LiAlH4 (29 mmol) were added to a dry three-necked flask and the reaction system was cooled to 0 °C. Subsequently, 4.9 g of D-alanine (55.0 mmol) was added to the reactor in batches, and the addition process lasted for 30 min. After the addition was completed, stirring was continued at 0 °C for 2 h. Then the temperature was gradually increased to reflux and the reflux reaction was maintained for 16 h. The reaction system was then cooled to 0 °C for 2 h. Upon completion of the reaction, the reaction system was cooled in an ice bath, and 100 mL of ether, 4.5 mL of water, 4.5 mL of 15% NaOH solution, and 12 mL of water were added slowly in sequence to quench the reaction. The reaction mixture was filtered through diatomaceous earth and the filtrate was collected. The filtrate was concentrated under reduced pressure to remove the solvent to give the crude product. The crude product was purified by silica gel column chromatography with the eluent of dichloromethane:ethanol=10:1 (v/v), resulting in 3.41 g of colorless oily product in 82.5% yield.
References
[1] Jeffery A, et al. Synthesis of Acyclic Nucleoside and Nucleotide Analogues from
Amino Acids: A Convenient Approach to a PMEA±PMPA Hybrid. Tetrahedron, 2000; 56: 5077-5083.