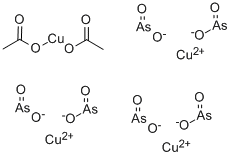
Chemical Properties
bluish-green powder
Chemical Properties
Paris green (copper acetoarsenite) is an
odorless emerald green crystalline powder which decomposes
upon heating.
Uses
Insecticide; wood preservative; as pigment, particularly for ships and submarines.
Preparation
Copper(II) acetoarsenite, Cu(C2H3O2)2·3Cu(AsO2)2, is a water-insoluble emerald green, poisonous powder. It is also known as paris green. Copper(II) acetoarsenite is produced by the reaction of a solution of copper(II) sulfate with arsenic(III) oxide, sodium carbonate, and acetic acid. It can also be prepared by reaction of copper(II) oxide with a hot solution of acetic acid and arsenic(III) oxide or by reaction of copper(II) acetate with arsenic(III) oxide. It has limited application as an antifouling pigment, in the preservation of wood, and as an insecticide.
Definition
Emerald: The green gem variety ofberyl: one of the most highly prizedgemstones. The finest specimensoccur in the Muzo mines, Colombia.Other occurrences include the UralMountains, the Transvaal in SouthAfrica, and Kaligunan in India. Emeraldscan also be successfully synthesized.
General Description
An emerald-green powder. Usually contains some water. Insoluble in water. Toxic by inhalation and by ingestion. Used as a wood preservative, insecticide, and pigment.
Air & Water Reactions
Insoluble in water. Decomposes on prolonged heating in water [Merck].
Reactivity Profile
Copper acetoarsenite is soluble in acids. Is unstable in acids, bases and toward hydrogen sulfide [Merck]. Has weak oxidizing or reducing powers. Redox reactions can however still occur.
Hazard
Toxic by ingestion.
Health Hazard
Copper acetoarsenite is extremely toxic; the probable oral lethal dose for humans is 5-50 mg/kg, or between 7 drops and 1 teaspoonful for a 150-lb. person. Some absorption may occur through the skin and by inhalation, but most poisonings result from ingestion. It may cause eye and respiratory tract irritation. Industrial exposure may cause dermatitis.
Fire Hazard
Poisonous, volatile arsenic oxide may be formed in fires. Exposure of dust to flame may cause explosion. When heated, or on contact with acid or acid fumes, Copper acetoarsenite emits highly toxic fumes. Decomposes readily in the presence of water and carbon dioxide to yield phytotoxic arsenical compounds. Can react vigorously with oxidizing materials. Unstable in acids and bases. Hazardous polymerization may not occur.
Safety Profile
Poison by ingestion and
possibly other routes. An insecticide. When
heated to decomposition it emits very toxic
fumes of As. See also ARSENIC
Potential Exposure
This material, and organoarsenic
compound, is used primarily as an insecticide; it may be
used as a wood preservative and a pigment, particularly for
ships and submarines; and also finds use as an anthelmintic.
Shipping
UN1585 Copper acetoarsenite, Hazard Class:
6.1; Labels: 6.1-Poisonous materials. STN: 49 232 20.
UN3465 Organoarsenic compound, solid, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required
Incompatibilities
Can react vigorously with oxidizers.
Emits highly toxic arsenic fumes on contact with acid or
acid fumes; and in elevated temperatures.
Waste Disposal
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations
governing storage, transportation, treatment, and waste
disposal. In accordance with 40CFR165, follow recommendations
for the disposal of pesticides and pesticide
containers. Must be disposed properly by following
package label directions or by contacting your local or federal environmental control agency, or by contacting
your regional EPA office.