Chemical Properties
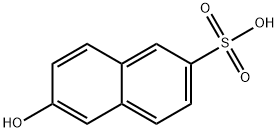
2-Hydroxynaphthalene- 6 -sulfonic acid [93-01-6]. (6-hydroxynaphthalene-2-sulfonic acid), Schaeffer acid, C10H8O4S, Mr 224.23, is readily soluble in water, from which it can be recrystallized as the monohydrate (mp 129℃). The sodium salt is sparingly soluble in cold water (1.7 %) but forms a 30 % solution at 80℃. Coupling with diazo compounds results in substitution in the 1-position as does treatment with bromine, nitrous acid, or oleum. Fusion with potassium hydroxide gives 2,6-dihydroxynaphthalene, amination gives 2- aminonaphthalene-6-sulfonic acid, and sulfonation (20 % oleum, 25℃) results in 2-hydroxynaphthalene-1,6-disulfonic acid.
Uses
2-Hydroxynaphthalene-6-sulfonic acid is used as a coupling component for a wide range of azo dyes, e.g., C.I. Acid Orange 12, C.I. Food Yellow 3, and C.I. Food Orange 2. It is also used as an intermediate for more highly substituted dye components and synthetic tanning agents. The 1-nitroso derivative forms an iron complex (C.I. Acid Green 1) which was formerly used as a wool dye.
Preparation
2-Naphthol is added to a mixture of sulfuric acid and sodium sulfate, and the reaction is continued at 90 C for 12 h. After pouring the reaction mixture into water, the sodium salt of the product is precipitated by addition of salt and filtered at 60℃ to separate the isomers.