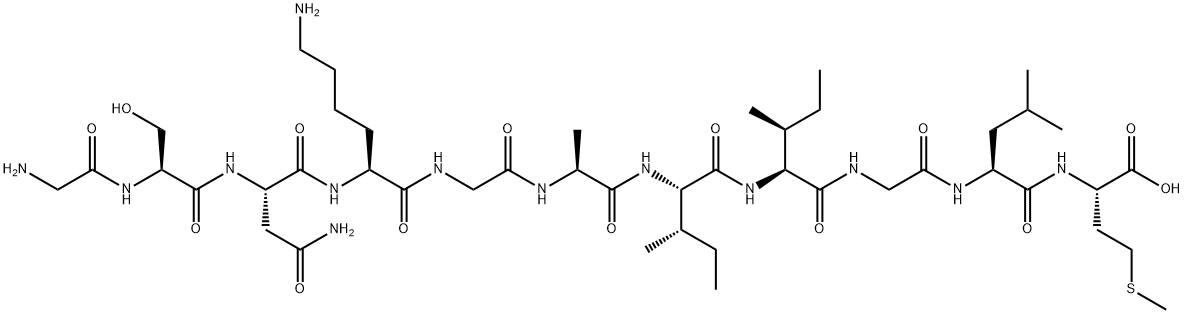
Description
Beta-amyloid protein (Abeta), a major component of senile plaques of Alzheimer's disease (AD) in the brain, causes elevation of the intracellular free Ca2+ level and the production of robust free radicals. Beta-amyloid 25-35 induced apoptosis, characterized by decreased cell viability, neuronal DNA condensation, and fragmentation, is associated with an increase in intracellular free Ca2+ level, the accumulation of reactive oxygen species (ROS), and the activation of caspase-3. All of these effects induced by beta-amyloid 25-35 are reversed by genistein.
Uses
Amyloid β-Protein Fragment 25-35 has been used:
- to induce neurotoxicity in cortical cultures
- to induce Alzheimer′s disease in rat model
- to induce apoptosis in mesenchymal stem cells (MSCs)
General Description
Amyloid β-Protein Fragment 25-35 (Aβ25-35) is derived from the amyloid-β protein.amyloid-β protein, which is mapped to human chromosome 21q21. Aβ25-35 lacks the N-terminal domain and the metal binding site and is majorly generated by proteolytic cleavage of Aβ(1?40) peptides. It has a β-sheet and β-turn structure.
Biological Activity
Amyloid β-peptide (25-35) (human) is a fragment of human amyloid β-peptide, functionally required for the neurotrophic and neurotoxic effects associated with Alzheimer's disease.
Biochem/physiol Actions
Amyloid β-Protein Fragment 25-35 (Aβ25-35) is involved in the pathogenesis of Alzheimer′s disease. Inhibitors of this transition may serve as a potential agent in managing Alzheimer′s disease. It is present in the subiculum and entorhinal cortex neurons of Alzheimer′s brain samples and inclusion-body myositis (IBM) muscle. It binds to receptors present in microglia and is capable of lipid membrane insertion. The functional domain sequence of Aβ comprising of sequence GSNKGAIIGLM elicits neurotrophic and neurotoxic effects. Aβ25-35 exhibits rapid aggregation and displays age dependant neurotoxicity.
Mechanism of action
Amyloid β (Aβ) peptide is a proven major contributing component of neuritic plaques of Alzheimer's disease (AD) . The formation of fibrillar deposits of Aβ peptide in brain is a key step in the pathogenesis of this disease, since the conversion of Aβ from soluble monomer to insoluble fibril is considered to cause the neuronal degeneration and clinical dementia in AD patients. Recent biophysical studies such as electron microscopy, solid-state NMR, Fourier transform infrared (FTIR), and electronic circular dichroism (ECD) spectra indicated that the Aβ fibrils exhibit a high β-sheet content. The conversion of normal Aβ peptides with water-soluble α-helical/random coil structures into the insoluble Aβ aggregates with an extensive β-sheet content is considered to be the predominant event in the onset of AD.