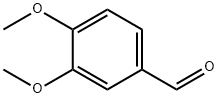
Chemical properties
White to brown or blue-gray needle crystal (crystallization in ether); sweet woody fragrance, fragrant grass aroma and strong flavor of vanilla blue; oxidized into odorless 3,4-dimethoxybenzoic acid (veratric acid) in the air; Melting point 43~45℃; Insoluble in cold water; soluble in hot water, ethanol and oil.
Natural products exist in the Java citronella oil and Hu Xanthium parsley oil and so on.
Use GB 2760 a 1996 provisions for the use of food spices.
Application
- This product is the intermediate of organic synthesis. In pharmaceutical industry, it is used to synthesize methamphetamine and also used in the production of veterinary drug sulfonamide synergist. Adding 0.02% (weight) of this veterinary drug into feed can increase the efficiency of the iodine drug synergistic added in feed, which can prevent and control the poultry bacterial infection.
- Used as pharmaceutical intermediate and mainly used for antibiotic drug synthesis.
Preparation
It can be derived from the reaction of 3-methoxy-4-hydroxybenzaldehyde (vanillin) and dimethyl sulfate, or derived from the methylation of dimethyl sulfate with vanillin in alkaline conditions.
Chemical Properties
off-white crystalline solid
Chemical Properties
Veratraldehyde has a very sweet, woody, vanilla-like odor with a warm, sweet, vanilla-like taste. This compound gets
oxidized in air to odorless veratric acid.
Chemical Properties
Veratraldehyde is a crystalline solid (mp 44.5–45°C) with a woody, vanillalike
odor.
Veratraldehyde can be prepared by methylation of vanillin. It is used in oriental
and warm, woody fragrances, as well as in flavor compositions for vanilla notes. It
is an intermediate in, for example, the synthesis of pharmaceuticals.
Occurrence
Reported among the constituents of the essential oils of Cymbopogon javanensis and Eryngium poterium. Also
reported found in raspberry, ginger, peppermint oil, Bourbon vanilla and mastic gum oil.
Uses
Veratraldehyde was used in the preparation of 4-chloromethyl-2-(dimethoxyphenyl)-1,3-dioxolane. It was used in the synthesis of (+)-lithospermic acid, having anti-HIV activity. It forms 1:1 inclusion complexes with cyclodextrins. It reacts with 3-acetyl-2,5-dimethythiophene to yield chalcone dye, (2E)-3-(3,4-Dimethoxyphenyl)-1-(2,5-dimethylthiophen-3-yl)prop-2-en-1-one.
Preparation
Prepared from vanillin by methylation of vanillin with dimethylsulfate under mildly alkaline conditions.
Definition
ChEBI: Veratraldehyde is a dimethoxybenzene that is benzaldehyde substituted by methoxy groups at positions 3 and 4. It is found in peppermint, ginger, raspberry, and other fruits. It has a role as an antifungal agent. It is a member of benzaldehydes and a dimethoxybenzene.
Aroma threshold values
Aroma characteristics at 1.0%: phenolic, sweet vanilla, powdery, slightly woody with a candy and cocoa
creaminess.
Taste threshold values
Taste characteristics at 50 ppm: phenolic, vanilla, sweet powdery cocoa, creamy, cherry-like with phenolic
and balsamic nuances..
Synthesis Reference(s)
Journal of the American Chemical Society, 69, p. 2070, 1947
DOI: 10.1021/ja01200a517Organic Syntheses, Coll. Vol. 2, p. 619, 1943
Tetrahedron Letters, 20, p. 975, 1979
General Description
Needles or chunky light peach powder. Has an odor of vanilla beans.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Veratraldehyde is incompatible with strong oxidizing agents and strong bases. . Veratraldehyde is an aldehyde. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction).
Fire Hazard
Veratraldehyde is probably combustible.
Flammability and Explosibility
Not classified
Biochem/physiol Actions
Taste at 50 ppm
Purification Methods
Crystallise the ether from diethyl ether, pet ether, CCl4 or toluene. [Beilstein 8 IV 1765.]