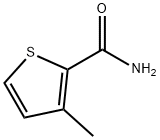
Preparation
3-Methyl-2-Thiophenecarboxamide synthesis: 1,2-Dichloroethane (280 mL), DMF (0.05 mL), 3-methyl-2-thiophenecarboxylic acid 5 (21.3 g, 0.149 mol), and SOCl2 (71.4 g, 43.8 mL, 0.6 mol) were heated at reflux temperature for 2 h with stirring. The solvent was then removed under vacuum to give 24 g of 3-methyl-2-thiophenecarbonyl chloride. This product was dissolved in 100 mL of CH2Cl2 and added to a solution of concentrated NH4OH (110 mL) in 300 mL CH2Cl2 with ice/salt bath cooling, at such a rate so as to maintain the reaction temperature below –5°C. The mixture was stirred for 20 min, separated, and the aqueous phase was extracted with another 200 mL of CH2Cl2. The combined organic layers were washed with water, then brine, and the solvent removed to give 21.2 g of 3-methyl-2-thiophenecarboxamide 15 as an off white solid, m.p. 123-124°C; 99.6% pure by GC area %; 1H NMR (CDCl3) 7.3 (d, 1H), 6.9 (1H), 5.8 (s, b), 2.5 ppm (s, 3H).