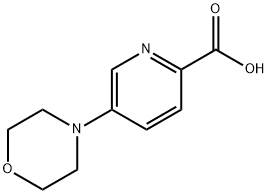
Synthesis
4-[6-(piperidin-1-ylcarbonyl)pyridin-3-yl]morpholine (18.3 g) was added to 25% aqueous KOH solution (100 mL) and the mixture was heated to reflux. After the reaction was completed, the reaction solution was neutralized with HCl. Subsequently, the solution was concentrated under reduced pressure to remove the solvent. The product was purified by hot isopropanol extraction to give finally (5-morpholin-4-yl)pyridine-2-carboxylic acid (11.5 g, 71% yield). The product was detected by ESI MS and showed [M+H]+ peak of 209.7; 1H NMR (400 MHz, DMSO-d6) δ ppm: 8.34 (br.s, 1H), 8.03 (d, 1H), 7.65 (dd, 1H), 3.75 (br.s, 4H), 3.40 (br.s, 4H).
References
[1] Patent: US2011/111046, 2011, A1. Location in patent: Page/Page column 15
[2] Patent: WO2011/58473, 2011, A1. Location in patent: Page/Page column 30
![Methanone, [5-(4-morpholinyl)-2-pyridinyl]-1-piperidinyl-](/CAS/20210305/GIF/1301214-68-5.gif)