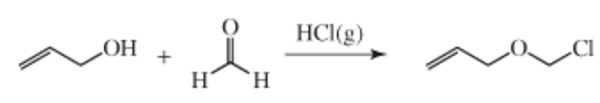Synthesis
Allylchloromethyl ether is prepared by bubbling anhydrous hydrogen chloride gas through an equimolar mixture of allyl alcohol and paraformaldehyde until the reaction completes (eq 1). The required quantity of the gas is generated by dripping concentrated sulfuric acid over dry lumps of sodium chloride (lumps of common salt are used efficiently and are usually dried by heating in an oven at 110?°C for a day). The gas obtained is passed through a wash bottle containing conc. H2SO4 and bubbled through the reaction mixture. The initial milky reaction mixture turns into a clear liquid with two separate layers. The upper organic layer contains crude product, while the lower aqueous layer consists of aqueous HCl. The organic layer is separated immediately and dried over sodium sulfate or magnesium sulfate. The crude allyl chloromethyl ether is distilled to provide a sufficiently pure reagent.
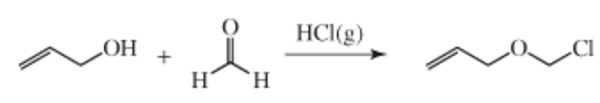
Solubility in organics
soluble in toluene, hexanes, petroleum ether, diethyl ether, CH2Cl2, acetone, MeOH, EtOH, and MeCN; partially soluble in water