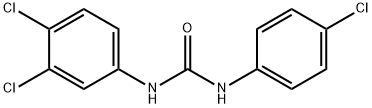
Chemical Properties
white crystals or powder
Originator
Cutisan,Innothera,France,1960
Uses
Used as bacteriostat and antiseptic in soaps and other cleansing compositions. Antiseptic, disinfectant.
Uses
Labelled Triclocarban
Definition
ChEBI: A member of the class of ureas that is urea substituted by a 4-chlorophenyl and a 3,4-dichlorophenyl group at positions 1 and 3 respectively.
Manufacturing Process
To a suitable reaction vessel equipped with a thermometer, agitator and reflux
condenser and containing 8.1 parts by weight (substantially 0.05 mol) of 3,4-dichloroaniline in approximately 57 parts by weight of diethyl ether is added
dropwise a solution of 7.7 parts by weight (substantially 0.05 mol) of 4-
chlorophenyl isocyanate in approximately 15 parts by weight of diethyl ether
at such a rate so as to maintain gentle reflux. Upon completion of the
isocyanate addition the reaction mass is agitated for about one hour. The
mass is filtered and the residue washed with diethyl ether. The dried product
is a white fluffy solid which on recrystallization from ethanol gives fine white
plates of 4,3',4'-trichlorocarbanilide, melting point 255.2°C to 256.0°C (88.0%
yield)
Therapeutic Function
Disinfectant
General Description
Fine white plates or white powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A halogenated amide. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Fire Hazard
The flash point of Triclocarban has not been determined. Triclocarban is, however, probably combustible.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
3,4,4′-Trichlorocarbanilide interferes with mammalian reproduction and can cause methemoglobinemia in humans.
Safety Profile
Moderately toxic byintraperitoneal route. When heated to decomposition itemits very toxic fumes of Clí and NOx.