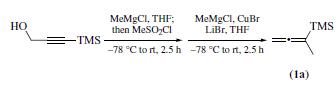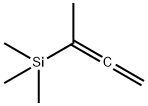
1-METHYL-1-(TRIMETHYLSILYL)ALLENE
- Product Name1-METHYL-1-(TRIMETHYLSILYL)ALLENE
- CAS74542-82-8
- CBNumberCB7184217
- MFC7H14Si
- MW126.27
- MDL NumberMFCD01321223
- MOL File74542-82-8.mol
- MSDS FileSDS
Chemical Properties
| Boiling point | 111-112 °C(lit.) |
| Density | 0.759 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point | 19 °F |
| solubility | sol CH2Cl2, benzene, THF, Et2O, and most organic solvents. |
| FDA UNII | T7C73V8VZJ |
Safety
| Symbol(GHS) |

|
| Signal word | Danger |
| Hazard statements | H225 |
| Precautionary statements | P210 |
| Hazard Codes | F |
| Risk Statements | 11 |
| Safety Statements | 16 |
| RIDADR | UN 1993 3/PG 2 |
| WGK Germany | 3 |