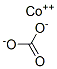Chemical Properties
This occurs naturally as sphaerocobaltite. It can be precipitated
as the violet-red hexahydrate CoCO3. 6H2O from aqueous solutions of cobalt(II) salts and
an alkali metal bicarbonate if the reaction is carried out under an atmosphere of carbon
dioxide. In the absence of carbon dioxide basic salts are precipitated in these reactions.
The hydrate is dehydrated to the CoCO3 at 140° and at 350° in vacuo CoO is formed.
Chemical Properties
Red crystals.
Insoluble in water and ammonia; soluble in acids.
The cobalt carbonate of commerce is usually the
basic salt (see following entry).
Uses
Ceramics, trace element added to soils and animal feed, temperature indicator, catalyst, pigments.