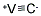Structure
Dark, very hard, chemically resistant submetallic substances. The V-C system has two phases:
VC: Homogeneity region VC
0.92-VC
0.74; m.p. 2800°C. Crystal structure: B 1 (NaCl) type.
V2C: Homogeneity region VC
0.4-VC
0.5. Crystal structure: L 3 type
Chemical Properties
Crystal. Mohs hardness 2800 kg/sq mm,
resistivity 150 μΩ?cm
(room temperature).
Physical properties
Black crystals soluble in HNO
3with decomposition. Wear resistant film, cutting tools.
Resistant to oxidation in air up
to 300°C.
Uses
Alloys for cutting tools, steel additive.
Uses
Vanadium carbide (VC) is used to alloy iron to produce high-speed, high-temperature cutting
tools for cutting metals and other hard substances.
Uses
Vanadium carbide (VC) and niobium carbide (NbC) are also extremely hard refractory materials. VC can be used as an additive to tungsten carbide (WC) to refine the carbide crystals and to improve the property of the cermet. TiC, ZrC, VC and NbC are commercially used in tool bits for cutting tools.
Production Methods
V + C = VC
The reactants, preferably in finely subdivided form, are intimately mixed and, if needed, also compressed into pellets. The reaction is then carried out in a high vacuum. At a temperature of 1300°C, approximately 24 hours, or at 2000 ℃ about 15 minutes, are required for homogenization of the product. The reaction is best carried out in a graphite crucible.
Flammability and Explosibility
Non flammable