Properties
Mr approx. 32,000 (mature inhibin). Additional Mr
forms of 55,000–105,000 exist due to the processing of the
precursor (Mr 105,000).5 pI 6.9–7.3. Stable in 8M urea. Dissociated to two subunits in 2% (v/v) 2-mercaptoethanol
Gene, mRNA, and precursor
The inhibin α- and β-subunits are encoded by separate
genes. The human inhibin α-subunit gene, INHA, location
2q35, consists of two exons. The α-subunit mRNA has
1098 b that encode a precursor comprised of three
domains: the prodomain, the αN domain, and the αC
domain. The human βA-subunit gene, INHBA, location
7p14.1, consists of two exons. The human βB-subunit
gene, INHBB, location 2q14.2, consists of two exons.
The INHBA and INHBB mRNAs have 1278 and 1221 b,
respectively. The precursor β-subunits consist of a prodomain at the N terminus and a mature βA or βB domain at
the C terminus. The coexpression of inhibin α- and β-subunit genes
suggests the production of inhibin molecules. In females,
mRNAs for α-, βA-, and βB-subunits are localized in the
granulosa cells of mammals, birds, and fish. mRNAs
for α- and βA-subunits are localized in the luteal cells of
humans and primates and in the placenta of mice and
humans. In males, α-, βA-, and βB-subunit mRNAs are
localized in Sertoli cells and Leydig cells. These mRNAs
are also detected in the adrenal cortex.
Synthesis and release
FSH, cAMP, and forskolin stimulate the secretion of
inhibins from granulosa cells and Sertoli cells in various
mammals. Inhibin production from granulosa cells is
suppressed by the epidermal growth factor (EGF).
Inhibin production in primate and human luteal cells is
promoted by the luteinizing hormone (LH) and human
chorionic gonadotropin (hCG). Adrenocorticotropic hormone (ACTH) stimulates inhibin secretion from adrenal
cortex cells. In the chicken, theca cell-derived bone morphogenetic protein (BMP) stimulates inhibin production
from granulosa cells.6 In zebrafish, oocyte-derived BMP
stimulates inhibin production from ovarian follicle cells.
Receptors
A specific receptor for inhibin has not been identified.
It is now accepted that inhibin actions result from the
antagonism of activin signaling in the presence of betaglycan. Human betaglycan (type III TGF-β receptor) has
851 aa residues that are expressed on the surface of pituitary cells, granulosa cells, theca cells, Sertoli cells, Leydig
cells, and adrenal cortex cells. It consists of an extracellular domain, a transmembrane region, and a short intracellular domain but lacks a signaling domain. Inhibins bind
to activin type II receptors via their β-subunits, and to
betaglycan via the α-subunit to form a stable complex.
The complex occupies activin type II receptors and prevents activin from activation of the type I receptors,
resulting in a blockade of the Smad 2/3 signaling pathway.
Biological functions
Inhibins suppress the expression of the FSH β-subunit
in the pituitary, and thereby regulate gonadal functions
and development. In addition, inhibins have paracrine
and autocrine effects in various cells. Inhibin α-subunit gene knockout mice develop Sertoli
cell tumors in males and granulosa cell tumors in
females. When the knockout mice were gonadectomized, the life expectancy increased; however, these mice
developed adrenal tumors around 30 weeks of age.
Inhibin βA-subunit gene knockout mice die perinatally
and have defects in tooth, palate, and retinal formation.
Clinical implications
From the findings that inhibin α-subunit knockout
mice develop gonadal and adrenal tumors, the α-subunit
gene is expected to act as a tumor suppressor gene. However, no consistent gene mutations have been identified
in cancer patients. Correlated with tumor growth, inhibin
production is enhanced in several types of adrenal and
gonadal tumors. Women affected with premature ovarian failure show low serum levels of inhibin A and
inhibin B. A decrease in testicular inhibin B production
is noted in men with testicular dysfunction. Pregnancies
affected with Down’s syndrome accompany high circulating concentrations of inhibin A. Hyperplasia of the
adrenal cortex (Cushing’s syndrome) often raises inhibin
A secretion from the adrenal gland.
Description
Inhibin is a glycoprotein, a member of the transforming
growth factor (TGF)-β superfamily. Inhibin is secreted
mainly from the gonads and inhibits follicle-stimulating
hormone (FSH) secretion from the anterior pituitary and
in turn regulates gonadal function and development. The presence of FSH-inhibiting activity had been
reported in the gonads since the 1970s, and the FSHinhibiting factor “inhibin” was first isolated in 1985 from
porcine1 and bovine follicular fluid.
Clinical Use
Inhibin levels in the circulation are a reliable marker
for granulosa cell tumors and serous and mucinous epithelial carcinomas. This may be true for granulosa cell
tumors in mares. Evaluation of plasma inhibin A levels
until the second trimester of pregnancy is useful for
screening for Down’s syndrome. The circulating inhibin
B is a good predictor for the conditions of spermatogenesis. High levels of inhibin are noted in humans, rats, and
dogs with Leydig cell tumors, whereas a low level clinically suggests a premature ovarian failure.
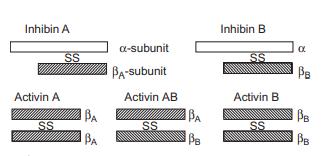
Structure and conformation
Inhibins and activins are structurally related glycoprotein hormones. Inhibins are disulfide-linked heterodimers
composed of an α-subunit and either a βA-subunit (inhibin
A) or a βB-subunit (inhibin B), whereas activins are homodimers or heterodimers made up of βA- and βB-subunits. The monomeric α-subunit, devoid of FSHsuppressing activity, has also been identified in follicular
fluid. The α-subunit has N-linked glycosylation sites and
their degree of glycosylation modifies biological activity. An approximate 80% identity is seen in the sequences of
the human, porcine, bovine, and rat α-subunits . The mature βA-subunit shares the same aa sequence
among the above species while the βB-subunit
shows an approximate 90% identity. The human βA- and βB-subunits share 64% aa sequence
identity.