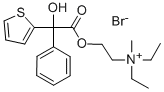
Manufacturing Process
Phenyl-(α-thienyl)hydroxyacetic acid (4.86 g), 2-diethylaminoethyl chloride (2.85 g) and 75 ml of isopropyl alcohol were refluxed for 15 h. After the addition of 50 ml of absolute alcohol to the cold mixture, it was treated with Norite (activated charcoal) at room temperature, filtered, and the solvents removed under reduced pressure. The residue crystallised when triturated with absolute ether. After recrystallisation from absolute alcohol, the phenyl(α-thienyl)hydroxyacetate of 2-diethylaminoethyl hydrochloride melted at 181182°C.
The producing of phenyl-(α-thienyl)hydroxyacetate of 2diethylmethylaminoethyl bromide may be carried out by methylation of phenyl-(α-thienyl)hydroxyacetate of 2-diethylaminoethyl hydrochloride with methylbromide.