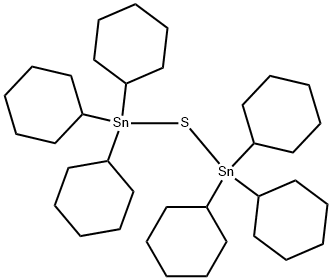
Uses
BIS(TRICYCLOHEXYLTIN) SULFIDE functions as a conveniently prepared in situ form of boron trisulfide; a powerful sulfurating agent for the conversion of carbonyl units into their corresponding thiono carbonyl analogs, e.g. thioaldehydes, thioketones, β-thiolactams, thiolactams; thiophenes; the analogous bis(tricyclohexyltin) selenideboron trichloride reagent is useful for selenation of carbonyl compounds to selenoaldehydes and selenoketones.
Preparation
BIS(TRICYCLOHEXYLTIN) SULFIDE is prepared in quantitative yield by treating tricyclohexyltin chloride with Na2S.9H2O in refluxing ethanol (95%) for 2 h; recrystallization from CH2Cl2/MeOH affords pure material, mp 130-132 °C.