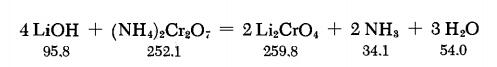Chemical Properties
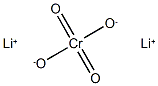
Lithium chromate is a yellow or orange-yellow crystalline powder. It dissolves in strong acids and alkalis but is insoluble in water and oil. It is made from lithium hydroxide and chromic acid.

Uses
Lithium Chromate is used in preparation method method of permeable crystalline cement-based waterproof coating by using maifanstone composite high water-absorbing resin and its application in building.
Preparation
According to the following equations, Li2CrO4 is formed on boiling a solution of (NH4)2Cr2O7 with LiOH.
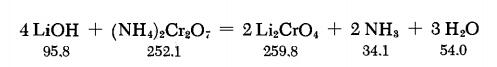
General Description
Lithium chromate appears as a yellow crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. It is used as a corrosion inhibitor and in the manufacture of other chemicals.
Air & Water Reactions
Deliquescent. Water soluble
Reactivity Profile
Lithium chromate is an oxidizing agent. Can oxidize combustibles [USCG, 1999]. Combining the chromate with zirconium can be explosive given the right proportions of reactants, [Z. Anorg., 1930, 191, 113].
Health Hazard
INHALATION: Corrosive to skin and mucous membranes causing dermatitis and slow healing ulcers. EYES: Conjunctivitis and lacrimation. INGESTION: Violent gastroenteritis, peripheral vascular collapse, vertigo, muscle cramps, coma, hemorrhagic diathesis, fever, liver damage and renal failure.
Potential Exposure
Used as a corrosion inhibitor, heat
transfer agent; and oxidizing agent in leather and metal finishing. Also used in photography, wood preservatives; batteries, safety matches, and cement
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. A regulated, marked area should be establishedwhere this chemical is handled, used, or stored in compliance with OSHA Standard 1910.1045. Store in tightlyclosed containers in a cool, well-ventilated area away from acids, hydrazine, chromic acid, combustible materials, sulfur, aluminum, plastics, and reducing agents.
Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
Incompatibilities
Aqueous solution is caustic. A strong
oxidizer; strong reaction with reducing agents, combustibles, organic material, hydrazine, chromic acid; sulfur,
acids. Attacks plastics and aluminum
References
[1] YINGCHUN LYU. Atomic insight into electrochemical inactivity of lithium chromate (LiCrO2): Irreversible migration of chromium into lithium layers in surface regions[J]. Journal of Power Sources, 2015, 273 1: 1218-1225. DOI:
10.1016/J.JPOWSOUR.2014.09.082.
[2] N.K. SHUKLA. Thermochemistry of lithium chromate Li2CrO4(cr) and lithium molybdate Li2MoO4(cr)[J]. Journal of Chemical Thermodynamics, 1992, 24 9: Pages 897-903. DOI:
10.1016/S0021-9614(05)80001-1.