Description
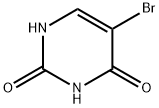
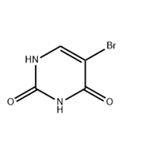
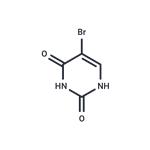
5-bromouracil (5-BU) is a uracil derivative, and its 5' position of the pyrimidine ring is halogenated. If 5-BU is incorporated into DNA in place of thymine, the tautomeric shift will occur, and the 5-BU will base pair with guanine. After the replication, an A-T to G-C transition occurs. Furthermore, the presence of 5-BU within DNA increases the sensitivity of the molecule to UV light. 5-BU is structurally similar to thymine. Therefore, it is considered a thymine analog and base-pairs normally with adenine. It is rarely present in its enol form, where its base pairs with guanine. 5-Bromouracil treats neoplasms in the form of its nucleoside, 5-bromo-2-deoxy-uridine[1-2].
Chemical Properties
Prisms from H
2
O.
Uses
A major chemical mutagen. Incorporates into DNA, altering base-pair sequencing by replacing thymine
Definition
ChEBI: A pyrimidine having keto groups at the 2- and 4-positions and a bromo group at the 5-position.
General Description
White powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A halogenated amide. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Hazard
Moderately toxic, alters DNA by replacing
thymine.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition 5-Bromouracil emits very toxic fumes of bromide ion and NOx.
Fire Hazard
Flash point data for 5-Bromouracil are not available, but 5-Bromouracil is probably combustible.
Safety Profile
Moderately toxic by
intraperitoneal route. Experimental
reproductive effects. Mutation data
reported. When heated to decomposition it
emits very toxic fumes of Brand NOx.
References
[1] Bhagavan, N. and C. Ha. “Chapter 21 – Structure and Properties of DNA.” (2015): 381-400.
[2] Shen, Chang-Hui. “Nucleic Acid-Based Cellular Activities.” Diagnostic Molecular Biology 25 1 (2019): 27-57.