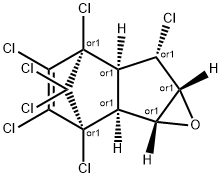
Description
Like pure heptachlor, heptachlor epoxide is awhite powder that does not explode easily. Heptachlor epox-ide is an oxidation product of heptachlor formed by plantsand animals, including humans, after exposure to heptachlor.About 20% of heptachlor is changed within hours intoheptachlor epoxide in the environment and in the body. It isalso present as acontaminant in heptachlor. It was notmanufactured and was not usedas an insecticide likeheptachlor. Molecular weight = 389.30; Freezing/Meltingpoint= 160- 162℃. Hazard Identification (based on NFPA-704 M Rating System): Health 3, Flammability 1, Reactivity0. Soluble in water.
Uses
The cis-metabolite of organochlorine pesticide Heptachlor.
Uses
Not known. A degradation product of heptachlor
Definition
A degradation product of heptachlor that also acts as
an insecticide.
General Description
Heptachlor epoxide is also a white powder. Bacteria and animals break down heptachlor to form heptachlor epoxide. The epoxide is more likely to be found in the environment than heptachlor. Heptachlor epoxide is a degradation product of heptachlor that occurs in soil and in or on crops when treatments with heptachlor, an insecticide, have been made. It forms readily upon exposing heptachlor to air. The U.S. EPA lists heptachlor epoxide as a possible human carcinogen.
Reactivity Profile
CIS-HEPTACHLOREPOXIDE EXO-, ISOMER B may react with acids, bases, and oxidizing and reducing agents.
Hazard
Possible carcinogen.
Health Hazard
ACUTE/CHRONIC HAZARDS: Toxic.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Safety Profile
Confirmed carcinogen
with experimental carcinogenic data. Poison
by ingestion and intravenous routes. Human
mutation data reported. When heated to
decomposition it emits toxic fumes of Cl-.
See also HEITACHLOR
Potential Exposure
Those involved in the manufacture,formulation, and application of this insecticide. Infants havebeen exposed to heptachlor and heptachlor epoxide throughmothers’milk, (cows’milk, and commercially preparedbaby foods. It appears that infants raised on mothers' milkrun a greater risk of ingesting heptachlor epoxide than ifthey were fed cows’ milk and/or commercially preparedbaby food. Persons living and working in or near heptachlortreated areashave a particularly high inhalation exposurepotential. Heptachlor epoxide has been found in at least 195EPA National Priorities List.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if con-taminated. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Source
When heptachlor is ingested by dairy animals, it is metabolized to heptachlor epoxide,
and stored in the fatty tissues. Heptachlor epoxide is present in the excreted milk and can be
present in other dairy products (Meyer et al., 1960).
Environmental Fate
Biological. In a model ecosystem containing plankton, Daphnia magna, mosquito larva (Culex pipiens quinquefasciatus), ?sh (Cambusia af?nis), alga (Oedogonium cardiacum) and snail (Physa sp.), heptachlor epoxide degraded to hydroxychlordene epoxide (Lu et al., 1975). Using settled domestic wastewater inoculum, heptachlor epoxide (5 and 10 mg/L) did not degrade after 28 days of incubation at 25°C (Tabak et al., 1981). This is consistent with the ?ndings of Bowman et al. (1965). They observed that under laboratory conditions, heptachlor epoxide did not show any evidence of degradation when incubated in a variety of soils maintained at 45°C for 8 days. The soils used in this experiment included Lakeland sand, Lynchburg loamy sand, Magnolia sandy loam, Magnolia sandy clay loam, Greenville sandy clay and Susquehanna sandy clay (Bowman et al., 1965). When heptachlor epoxide was incubated in a sandy loam soil at 28°C, however, 1hydroxychlordene formed at yields of 2.8, 5.8 and 12.0% after 4, 8 and 12 weeks, respectively (Miles et al., 1971).
Photolytic. Irradiation of heptachlor epoxide by a 450-W high-pressure mercury lamp gave two half-cage isomers, each containing a ketone functional group (Ivie et al., 1972). Benson et al. (1971) reported a degradation yield of 99% when an aceton
Graham et al. (1973) reported that when solid heptachlor epoxide was exposed to July sunshine for 23.2 days, 59.3% degradation was achieved. In powdered form, however, only 5 days were required for complete degradation to occur.
Chemical/Physical. Heptachlor epoxide will hydrolyze via nucleophilic attack at the epoxide moiety forming heptachlor diol which may undergo further hydrolysis forming heptachlor triol and hydrogen chloride (Kollig, 1993).
storage
Color Code- Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained (on its proper handling and storage.Store in tightly closed containers in a cool, well-venti latedarea away from ferrous metals. A regulated, markedareashould be established where this chemical is handled, used,or stored in compliance with OSHA Standard 1910.1045.