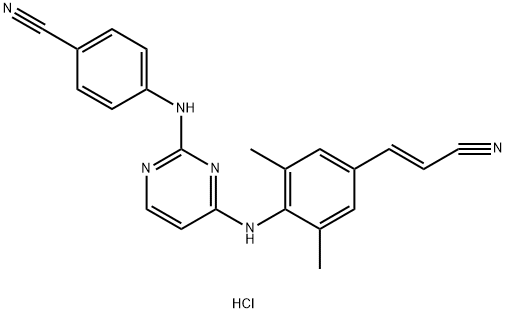
Uses
Rilpivirine Hydrochloride was shown to be able to treat and or prevent immunodeficiency virus-1. It also has uses for anti-viral therapy
Definition
ChEBI: A hydrochloride obtained by reaction of rilpivirine with one equivalent of hydrochloric acid. Used for treatment of HIV.
Clinical Use
Rilpivirine hydrochloride (Edurant), a non-nucleoside reverse
transcriptase inhibitor (NNRTI), received its approval both from
the U.S. FDA and E.U. EMA in 2011 for the treatment of HIV-1 infection in treatment-na?ve adult patients. It was discovered and
developed by Janssen Pharmaceuticals and its subsidiary Tibotec
Pharmaceuticals. As a second generation NNRTI, rilpivirine hydrochloride
displayed higher potency and longer half-life with a 25 mg
once a day dose, compared to existing NNRTIs, such as the 200 mg
BID of efavirenze (Sustiva). In late 2011, the fixed-dose
combination products of rilpivirine hydrochloride with two
nucleoside reverse transcriptase inhibitor (RTIs) emtricitabine and tenofovir disoproxil fumarate, co-developed by Gilead Science
and Tibotec, were also approved both by the FDA and EMA under
brand names Complera® and Eviplera®, respectively.
Synthesis
Similar
to efavirenze, rilpivirine hydrochloride is a diarylpyrimidine
(DAPY) compound, and the large-scale process synthesis begins
with commercially available 2-methylthio-4-pyrimidinone (193)
shown in the scheme.
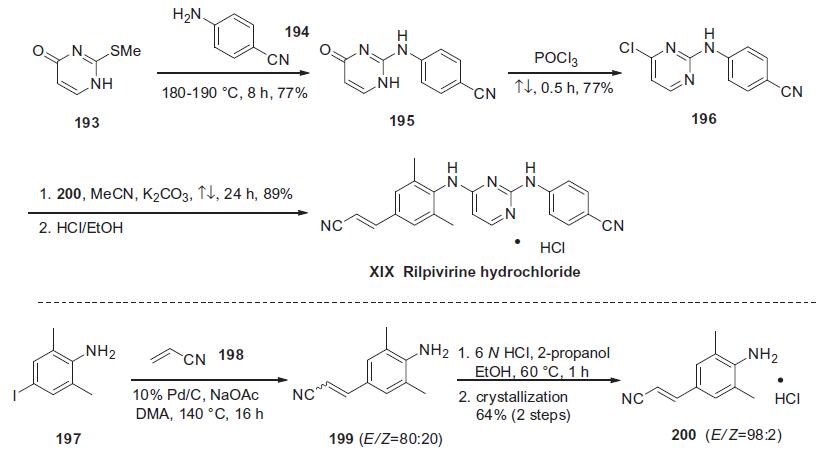
Thioether 193 was condensed with neat 4-cyanoaniline (194) at
elevated temperature to afford diarylamine 195 in 77% yield. Subsequent
treatment of pyrimidone 195 with refluxing POCl3 provided
the corresponding chloride 196 in 77% yield.160,161 In the
presence of K2CO3, chloride 196 was treated with the (E)-cinnamonitrile
aniline 200 to give rilpivirine hydrochloride (XIX) in good
yield.158 Aniline 200 was prepared via a Heck reaction of commercially
available 4-iodo-2,6-dimethyl-benzeneamine (197) and
acrylonitrile (198) affording compound 199 as a 4:1 mixture of E/Z isomers. The distribution of E/Z olefins was increased to 98:2
by salt formation and recrystallization to ultimately provide pure
(E)-200 in 64% yield for two steps.