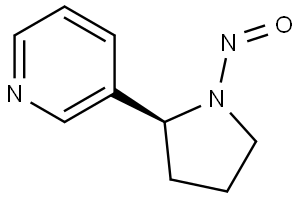
Description
A nicotine alkaloid, this base has been detected in some Japanese cigarette tobaccos and also in Kirsu tobacco where it occurs in the fresh leaves that have been dried without fermentation. From these two sources it has been isolated at 1.1 and 1. 8 ppm levels respectively
Chemical Properties
white crystals
Uses
A tobacco alkaloid as risk factor of human cancer.
General Description
White crystalline flakes or yellow oil that solidifies on standing in the cold.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A nitrated amine, nicotine derivative. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition it may emit toxic and hazardous fumes of NOx and COx.
Fire Hazard
Flash point data for N'-NITROSONORNICOTINE are not available; however, N'-NITROSONORNICOTINE is probably combustible.
Safety Profile
Confirmed carcinogen
with experimental carcinogenic,
neoplastigenic, and tumorigenic data. Low
toxicity by intraperitoneal route. Mutation
data reported. When heated to
decomposition it emits toxic fumes of NOx.
See also N-NITROSO COMPOUNDS.
Carcinogenicity
N-Nitrosonornicotine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
References
Bharadwaj et al., Gann., 66, 585 (1975)