Description
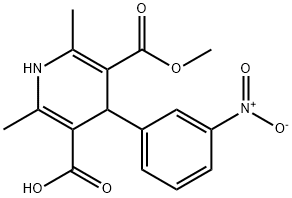
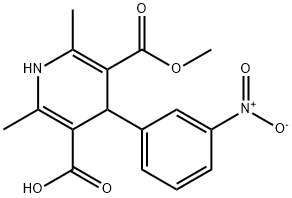
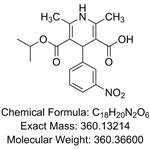
5-(Methoxycarbonyl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid(1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic Acid 3-Methyl Ester) is a pharmaceutical intermediate ingredient used in the preparation of Barnidipine, Lercanidipine hydrochloride and nitrendipine analogues. These drugs are all calcium channel blockers and are used to treat various forms of hypertension.
Chemical Properties
Light-Yellow Solid
Uses
1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic Acid 3-Methyl Ester can be used in the prevention and therapy of atherosclerotic degradation of arterial walls.
Uses
1,4-Dihydropyridine derivative used in the prevention and therapy of atherosclerotic degradation of arterial walls. Nicardipine USP Related Compound A.
Synthesis
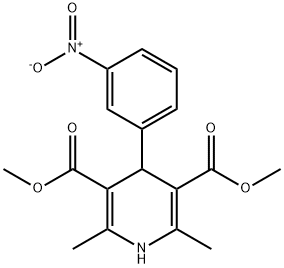
General procedure for the synthesis of monomethyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate from dimethyl 2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate:
(3) Preparation of 5-methoxycarbonyl-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid
Dimethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate (0.01 mol) was mixed with 150 mL of methanol. Saturated aqueous NaOH solution (9 g, 0.225 mol) was slowly added to the mixture under stirring conditions. The reaction mixture was vigorously stirred at 70 °C for 6 hours. Upon completion of the reaction, about 80 mL of methanol was removed by evaporation under reduced pressure. 200 mL of water was added to the residue and unreacted feedstock was removed by filtration. The filtrate was adjusted to pH about 2.5 with 1 mol/L hydrochloric acid, at which point a yellow solid precipitated, which was filtered and dried to give a khaki colored powder. The resulting powder was recrystallized from methanol to give the title product 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylic acid monomethyl ester as a yellow solid (1.9 g, 57.2% yield).
References
[1] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 3, p. 805 - 808
[2] Patent: US2014/45896, 2014, A1. Location in patent: Paragraph 0071; 0072
[3] Patent: EP2703398, 2014, A1. Location in patent: Paragraph 0067; 0068
[4] Patent: CN106279000, 2017, A. Location in patent: Paragraph 0032; 0033; 0034; 0035; 0036; 0037; 0038
[5] European Journal of Medicinal Chemistry, 2010, vol. 45, # 9, p. 3986 - 3992