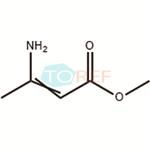
Chemical Properties
Crystals, yellowish-white
Uses
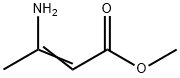
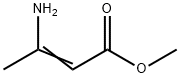
Methyl 3-aminocrotonate (Methyl 3-amino-2-butenoate) was used to synthesize 4-aryl-1,4-dihydropyridines possessing potential calcium channel blocking activity.
Uses
Methyl 3-aminocrotonate is used as intermediate for the syntheses of pharmaceuticals (e.g. 1,4-dihydropyridine derivatives) as well as for the manufacture of stabilizers and plastics. Product Data Sheet
Uses
Nitrendipine (N490150) metabolite. The pharmacokinetics of Nitrendipine was studied in the rat and 6 major metabolites were identified.
Application
Methyl 3-aminocrotonate reacts with benzene to form the corresponding E-2-phenyliodonio tosylate in good yield. This new alkenyl iodonium salt upon reaction with various nucleophiles offers an easy access to substituted enamine derivatives of crotonic acid. The reaction can be also extended to N-substituted crotonates
[1].
Preparation
3-Aminocrotonate Esters, e.g., methyl 3- aminocrotonate [14205-39-1] (3-amino-2-butenoic acid, methyl ester) can be prepared by treatment of acetoacetates with aqueous ammonia. They are mainly used in the production of a series of the dihydropyridine-type calcium antagonists.
General Description
Methyl 3-aminocrotonate undergoes waste-free solid-state cascade reaction with crystalline ninhydrin.
Flammability and Explosibility
Not classified
Synthesis
The general procedure for the synthesis of methyl 3-aminocrotonate from methyl acetoacetate was as follows: 25 kg of methyl acetoacetate and 20 kg of methanol were pumped into an ammoniation reactor. The temperature of the reaction system was controlled in the range of 0-10°C using an ice-water bath with continuous stirring, and the ammonia was slowly introduced until white crystals were precipitated, and then the introduction of ammonia was stopped. The reaction mixture was allowed to stand under refrigeration overnight and then centrifuged to collect the white crystals. The resulting white crystals were transferred to an ammonia refining kettle and purified by adding 15 kg of methanol, heating the mixture until complete dissolution, followed by cryocrystallization. The mixture was centrifuged again and filtered to collect the filter cake. The filter cake was placed in a hot air circulating oven and dried at 50-60 °C for 8 hours to give 18.6 kg of methyl 3-aminocrotonate. The product needs to be stored at 0 to -10°C for 20 to 24 hours. The yield of this process was 21 kg with a weight yield range of 74.4-84.0% and a molar yield range of 75.0-85.0%.
References
[1] IOANNIS PAPOUTSIS; Anastasios V ?; Spyros Spyroudis ?. Reactivity of a new alkenyl phenyliodonium tosylate derived from methyl 3-aminocrotonate[J]. Tetrahedron, 1998. DOI:10.1016/S0040-4020(97)10354-4.