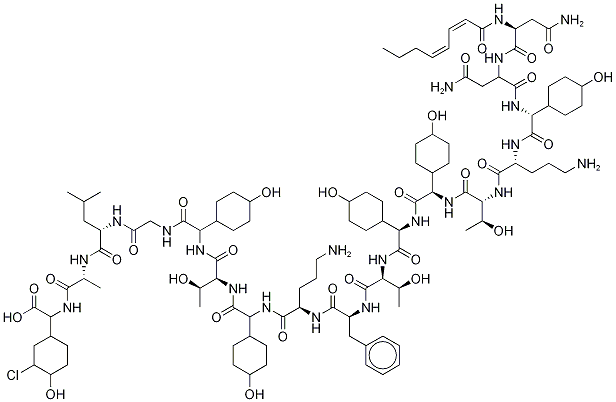
Ramoplanin
- Product NameRamoplanin
- CAS76168-82-6
- CBNumberCB61074884
- MFC106H170ClN21O30
- MW2254.0597
- MDL NumberMFCD01775776
- MOL File76168-82-6.mol
Chemical Properties
| Melting point | 210-230° |
| alpha | D20 +78.3° (c = 1.04 in H2O) |
| storage temp. | -20°C |
| solubility | H2O: soluble10mg/mL |
| form | powder |
| color | White to Off-White |
| InChIKey | FSBZBQUUCNYWOK-KFTDLNJOSA-N |
| EWG's Food Scores | 1 |
| FDA UNII | 0WX9996O2G |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H318 | |||||||||
| Precautionary statements | P280-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 41 | |||||||||
| Safety Statements | 26-39 | |||||||||
| WGK Germany | nwg | |||||||||
| RTECS | VE5050000 | |||||||||
| Toxicity | LD50 in mice (mg/kg): 328 i.p., 122 i.v.; orally in rats: 2000 mg/kg (Pallanza) | |||||||||
| NFPA 704: |
|
