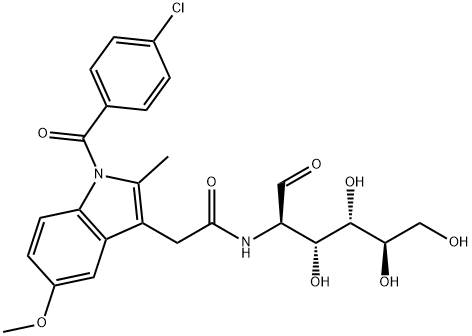
glucametacin
- Product Nameglucametacin
- CAS52443-21-7
- CBNumberCB5885480
- MFC25H27ClN2O8
- MW518.94
- EINECS257-923-9
- MDL NumberMFCD01725278
- MOL File52443-21-7.mol
Chemical Properties
| Melting point | >160°C (dec.) |
| Boiling point | 793.7±60.0 °C(Predicted) |
| Density | 1.44 |
| storage temp. | Refrigerator, under inert atmosphere |
| solubility | DMSO (Slightly) |
| form | Solid |
| pka | 13.05±0.20(Predicted) |
| color | White to Pale Yellow |
| FDA UNII | N1EXE5EHAN |
