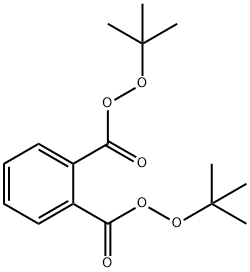
Reactivity Profile
Peroxides, such as DI-(TERT-BUTYLPEROXY)PHTHALATE, are good oxidizing agents. Organic compounds can ignite on contact with concentrated peroxides. Strongly reduced material such as sulfides, nitrides, and hydrides may react explosively with peroxides. There are few chemical classes that do not at least produce heat when mixed with peroxides. Many produce explosions or generate gases (toxic and nontoxic). Generally, dilute solutions of peroxides (<70%) are safe, but the presence of a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium) as an impurity may even then cause rapid decomposition, a buildup of heat, and even an explosion. Solutions of peroxides often become explosive when evaporated to dryness or near-dryness.
Purification Methods
Crystallise the perphthalate from Et2O or pet ether and dry it over H2SO4. The IR has max 1772cm-1 in CCl4. [Milas & Surgemor J Am Chem Soc 68 642 1946, Milas & Kelin J Org Chem 36 2900 1971, Beilstein 9 III 4190, 9 IV 3260.] Potentially EXPLOSIVE.