Synthesis
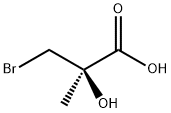
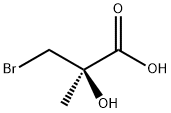
General procedure for the synthesis of (R)-3-bromo-2-hydroxy-2-methylpropionic acid (R-131) from the compound (CAS: 106138-80-1): bromolactone R-130 (18.5 g, 71 mmol) was dissolved in 300 mL of 24% hydrobromic acid and heated to reflux for 1 hour. After completion of the reaction, the resulting solution was diluted with 200 mL of brine and extracted with ethyl acetate (100 mL × 4). The organic phases were combined and washed with saturated sodium bicarbonate solution (100 mL × 4). The aqueous phase was acidified to pH = 1 with concentrated hydrochloric acid and extracted again with ethyl acetate (100 mL × 4). The organic phases were combined, dried with anhydrous sodium sulfate, filtered through diatomaceous earth, and concentrated to dryness under reduced pressure. The product was recrystallized from toluene to give 10.2 g (86% yield) of the target compound as colorless crystals. Melting point: 107-109 °C (S-isomer, literature value: melting point 109-113 °C).1H NMR (300 MHz, DMSO-d6) δ 3.63 (d, J=10.1 Hz, 1H, CHHa), 3.52 (d, J=10.1 Hz, 1H, CHHb), 1.35 (s, 3H, Me).IR (KBr) 3434 (OH), 3300-2500 (COOH), 1730 (C=O), 1449, 1421, 1380, 1292, 1193, 1085 cm-1. [α]D26 +10.5° (c=2.6, MeOH). Calculated elemental analysis (C4H7BrO3): C 26.25, H 3.86; measured values: C 26.28, H 3.75.
References
[1] Patent: US2003/225040, 2003, A1. Location in patent: Page 16
[2] Patent: US2006/9488, 2006, A1. Location in patent: Page/Page column 52
[3] Patent: WO2007/27582, 2007, A2. Location in patent: Page/Page column 61
[4] Patent: WO2007/62230, 2007, A2. Location in patent: Page/Page column 149; 151
[5] Patent: WO2008/8433, 2008, A2. Location in patent: Page/Page column 123; 1/19; 3/19; 7/19