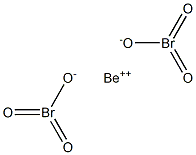
Preparation
Beryllium Bromate can be prepared by a reaction of bromic acid or
sodium bromate:
Be(OH)2+ NaBrO3→Be(OH)BrO3+ NaOH
The most stable method to produce this salt involved
the use of sodium bromate and HBr gas:
3Be(OH)2 (solid)+ 2NaBrO3 (aq)+ HBr (gas)
→3Be(OH)BrO3 (aq)+ 2NaOH (aq)+ 4H2O
Nevertheless, there are no reports of the preparation
of this salt in the technical literature and any such
description of its physical and chemical properties
remains a conjecture. It is not offered commercially nor
does it have a CAS number.