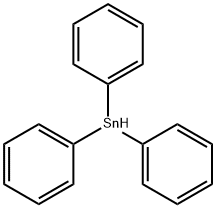
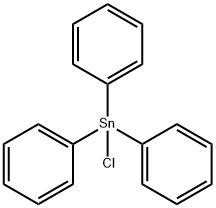
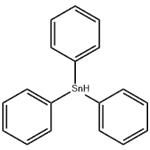
Chemical Properties
opalescent colorless or milky white to pale yellow. Soluble in many common organic solvents; usually used in benzene or toluene.
Uses
Although the
triphenyltins exhibit some curative effect, they are used
mainly as protectants for control of a range of diseases,
examples being early and late blights of potato and leaf
spots on sugar beet and peanut.
Reactivity Profile
Triphenylstannane could be used in the reactions:
Generation of carbon radicals from selenides, halides, sulfides, and alkynes; radical deoxygenation; reduction of α,β- unsaturated aldehydes and ketones; hydrostannylation of alkynes and alkenes; reversible addition of stannyl radicals to double bonds; formation of metal triphenylstannates; reduction of aldehydes and ketones; desulfonation of β-keto phenyl sulfones; hole-transfer-promoted hydrogenation.
reaction suitability
reagent type: reductant
Synthesis
The Preparative Method of Triphenylstannane: reduction of Chlorotriphenylstannane with Lithium Aluminum Hydride in ether or with Sodium Borohydride in glyme; reduction of bis(triphenyltin) oxide with polymethylhydrosiloxane; reduction of triphenyltin methoxide with diborane; protonation of Ph3SnLi. The reagent was also generated in situ from Ph3SnCl and Sodium Cyanoborohydride in t-butyl alcohol.
Precautions
Triphenylstannane (Ph3SnH) can be stored for several months and is easily repurified by Kugelrohr distillation (oil-pump vacuum) before use. Organotin hydrides decompose slowly at rt and are best stored at 0 °C or below.