Uses
Catalyst precursor in the interand intramolecular Pauson-Khand reaction.
Chemical Properties
black crystal(s); air sensitive [DOU83] [STR93]
Preparation
Tetracobalt dodecacarbonyl is most conveniently prepared by thermal decomposition
of the octacarbonyl at 50° in an inert atmosphere :
2Co2(CO)8 → Co4(CO)12+4CO
at present it cannot be prepared direct from cobalt(II) salts and carbon monoxide. Treatment of Co4(CO)12 with alkali metals in tetrahydrofuran gives the green-yellow [Co6(CO)15]
2-
ion which is slowly transformed into the red-brown [Co6(CO)14]
4- anion; these ions are
oxidized in aqueous solution by iron(III) chloride to a mixture of carbonyls from which
the black Co6(CO)16 can be extracted.
Structure and conformation
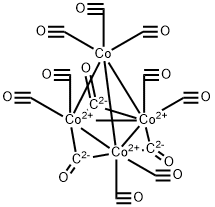
It consists of a tetrahedron of cobalt atoms ; three of these have two terminal CO groups and are bridged by CO groups, the other cobalt atom is bonded to three terminal CO groups and the other three cobalt atoms only. The infrared spectrum is considerably simpler than would be expected theoretically for such a structure and consequently a different structure has been proposed for Co4(CO)12 in solution. The carbonyl CO6(CO)16 has a similar infrared spectrum to, and is isomorphous with, Rh6(CO)16 so that it probably has the same structure.