Chemical Properties
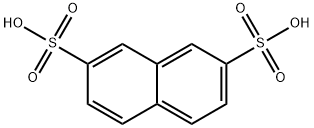
White, crystalline solid.Soluble in water. Combustible. Naphthalene-2,7-disulfonic acid [92- 41-1]. C10H8O6S2, Mr 288.28; the sodium salt of Naphthalene-2,7-disulfonic acid is much more soluble than that of the 2,6- isomer but can be isolated and purified by precipitation of the calcium salt at lower temperature followed by recrystallization.
Uses
Intermediate for dyes. The disodium salt of 8 gives 2-hydroxynaphthalene-7-sulfonic acid or 2,7-dihydroxynaphthalene by fusion processes. Sulfonation by oleum gives the 1,3,6-trisulfonic acid, and nitration gives 1-nitronaphthalene-3,6- disulfonic acid. In sulfuric acid solution, the disodium salt of naphthalene-2,7-disulfonic acid undergoes an unusual reaction at the 4-position with 4,40 -bis- (dimethylamino)benzhydrol. The resulting condensation product is oxidized with lead dioxide to give the acid basic dye C.I. Acid Green 16.
Production Methods
The 2,7-disulfonic acid is always the main product of high-temperature sulfonation of naphthalene, but at 160 C it converts slowly to an equilibrium mixture containing ca. 30 % of the 2,6-isomer. Crystallization of the pure product from conventional sulfonation is difficult, and alternative processes, (for example, reaction of naphthalene vapor with sulfur trioxide in the gas phase at 220 C) are said to give a purer product.
Definition
ChEBI: A naphthalenesulfonic acid in which the sulfo groups are attached to positions 2 and 7 of the naphthalene ring.
Purification Methods
Crystallise the acid from conc HCl. The disulfonamide has m 273o (from EtOH). [Beilstein 11 II 119, 11 III 463.]