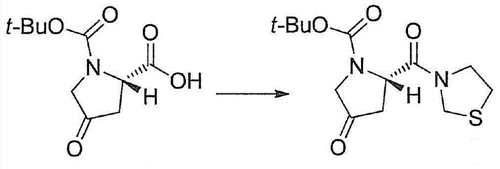
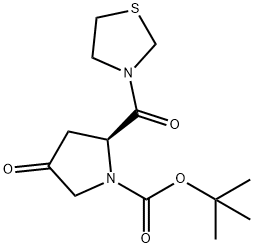
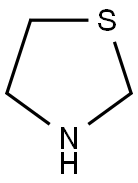
Synthesis
To (2S)-1-tertbutyloxycarbonyl-4-oxa-tetramethyleneimine-2-carboxylic
acid (60.0kg), thiazolidine (30.3kg) and the solution
of N-diisopropylethylamine (118kg) in ethyl acetate (595kg), adds the
solution of 28w% propyl phosphonous acid anhydride (cyclic trimer)
in ethyl acetate (446kg) at 2 ℃-7 ℃, and reaction mixture is stirred 2
hours at 2 ℃-4 ℃.To this reaction mixture, add 15w% aqueous citric acid
solution (600kg) for distributing, and ethyl acetate for water layer
(271kg) is extracted.
The ethyl acetate layer obtaining is mixed, and use
successively 10w% ammonium dibasic phosphate aqueous solution (600kg)
and water (300kg) washing. Ethyl acetate layer is concentrated into
residual quantity 300L, normal heptane (739kg), 23 ℃ of-25 ℃ of
interpolations, and is stirred this mixture 1 hour at 23 ℃-25 ℃, and
stir 2 hours at 1 ℃-5 ℃.
The crystal of precipitation is collected by
filtration, is used normal heptane (164kg) washing drying under reduced
pressure to produce (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester (67.8kg, yield 86%).