Chemical Properties
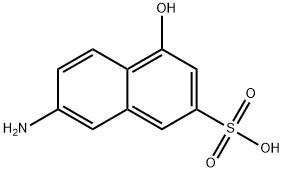
Gray needles, white when pure. Soluble in hot water; sparingly soluble in cold water. 2-Amino-5-hydroxynaphthalene-7-sulfonic acid [87-02-5]. (7-amino-4-hydroxynaphthalene-2-sulfonic acid), J acid, C10H9NO4S, Mr 239.2, is only slightly soluble (0.1 %) in cold water but readily soluble at 100℃. The alkalimetal salts are readily soluble in water. Diazo compounds are normally coupled with J acid under alkaline conditions with reaction in the 6-position (ortho to hydroxyl); however, under acid conditions, coupling can occur in the 1- position. Reaction with bisulfite gives 4,7-dihydroxynaphthalene-2-sulfonic acid. The N-acylation, Nalkylation, and N-arylation reactions are important.
Uses
Azo dye intermediate.
Production Methods
A concentrated disodium salt solution of 2-aminonaphthalene-5,7-disulfonic acid is added to 72 % sodium hydroxide at 150 ℃. After heating to 187℃ over 10 h reaction is continued at this temperature for a further 8 h until completion of the reaction is indicated by giving equivalent diazotization and coupling titers for a worked-up sample. Quenching, acidification, purging of sulfur dioxide, and filtration at 55 ℃ followed by washing with water at 50℃ result in an 88 % yield of paste. Purification by salting out the sodium salt from a hot alkaline solution gives a 90 % recovery of the product as gray crystals. An improved process with 90 % yield is claimed by feeding the concentrated 2-aminonaphthalene-5,7-disulfonic acid liquor and the caustic liquor simultaneously into the fusion reactor to maintain more stable conditions.
Definition
ChEBI: 7-amino-4-hydroxy-2-naphthalenesulfonic acid is an aminonaphthalenesulfonic acid that is 2-naphthalenesulfonic acid substituted by an amino group at position 7 and a hydroxy group at position 4 respectively. It has a role as a metabolite. It is an aminonaphthalenesulfonic acid and a member of naphthols.
Flammability and Explosibility
Not classified