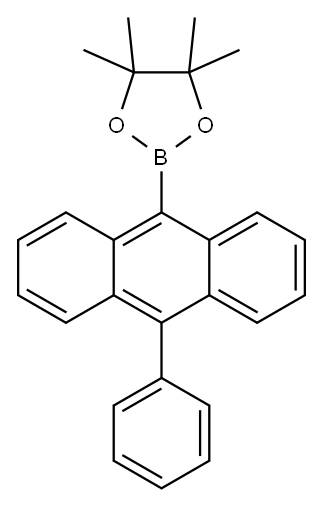
Preparation
9-Bromo-10-phenylanthracene (5.8g, 17.4mmol), bis(pinacolato)diboron(6.63 g, 26.1 mmol), Pd(dppf)Cl2 (710 mg, 0.87 mmol), and KOAc (10.23 g, 104.4 mmol) were mixed in a 250mL flask containing 1,4-dioxane (60 mL). The reaction mixture was refluxed for 24h under nitrogen. After it was cooled to room temperature, a dilute hydrochloric acid solution quenched. The mixture was extracted with CH2Cl2 and dried over anhydrous MgSO4. The crude product was concentrated by rotary evaporation and further purified by silica gel column chromatography (CH2Cl2 / petroleum ether (1:1,v/v)) to afford a light yellow powder (4.0 g, 60.4 %). 1H NMR (500 MHz, CDCl3) δ 8.46 (d, J = 8.7 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 7.57 (dq, J = 14.2, 7.0 Hz, 3H), 7.51 – 7.46 (m, 2H), 7.41 (d, J = 7.0 Hz, 2H), 7.34 (dd, J = 8.1, 7.1 Hz, 2H), 1.63 (s, 12H). EI-MS (m/z): Calculated for C26H25BO2: 380.29. Found [M+]: 379.92.
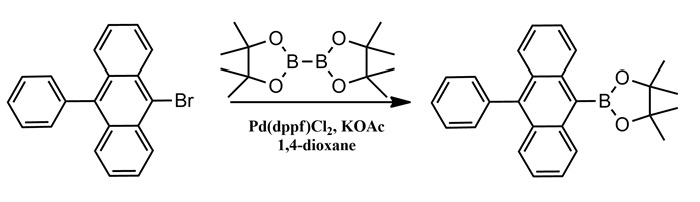
Synthesis of 4,4,5,5-Tetramethyl-2-(10-phenylanthracen-9-yl)-1,3,2-dioxaborolane